Oxybutynin Chloride (oxybutynin chloride 5 mg) Dailymed
Generic: oxybutynin chloride is used for the treatment of Urinary Bladder, Neurogenic Colitis, Ulcerative Intestinal Obstruction Megacolon, Toxic Myasthenia Gravis Ureteral Obstruction Urinary Incontinence Glaucoma, Angle-Closure Urinary Retention Gastroparesis Dysuria Glaucoma
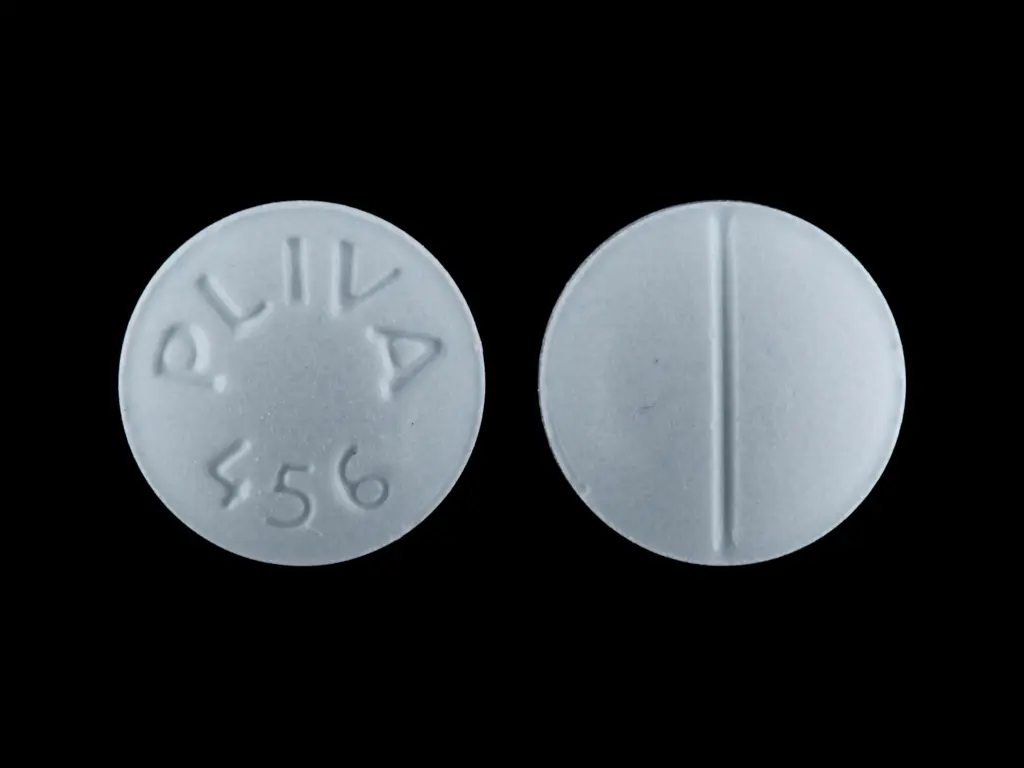
IMPRINT: PLIVA 456
SHAPE: round
COLOR: blue SCORE: 2
All Imprints
oxybutynin chloride 5 mg - pliva 456 round blue
oxybutynin chloride 5 mg oral tablet - pliva 456 round blue
Go PRO for all pill images
Description
Oxybutynin chloride is a white crystalline solid, readily soluble in water and acids, but relatively insoluble in alkalis. Chemically, it is the d,i-(racemic) form of 4-diethyl-amino-2-butynyl phenylcyclohexylglycolate hydrochloride with the following formula:
C22H32ClNO3M.W. 393.9
Each tablet for oral administration contains oxybutynin chloride, USP 5 mg.
Inactive ingredients include calcium stearate, microcrystalline cellulose, anhydrous lactose, sodium starch glycolate and FD&C Blue #1.
Clinical Pharmacology
Oxybutynin chloride exerts direct antispasmodic effect on smooth muscle and inhibits the muscarinic action of acetylcholine on smooth muscle. It exhibits only one-fifth of the anticholinergic activity of atropine on the rabbit detrusor muscle, but four to ten times the antispasmodic activity. No blocking effects occur at skeletal neuromuscular junctions or autonomic ganglia (antinicotinic effects).
Oxybutynin relaxes bladder smooth muscle. In patients with conditions characterized by involuntary bladder contractions, cystometric studies have demonstrated that oxybutynin increases bladder (vesical) capacity, diminishes the frequency of uninhibited contractions of the detrusor muscle, and delays the initial desire to void. Oxybutynin thus decreases urgency and the frequency of both incontinent episodes and voluntary urination.
Oxybutynin chloride was well tolerated in patients administered the drug in controlled studies of 30 days duration and in uncontrolled studies in which some of the patients received the drug for two years. Pharmacokinetic information is not currently available.
Indications And Usage
Oxybutynin chloride tablets are indicated for the relief of symptoms of bladder instability associated with voiding in patients with uninhibited neurogenic or reflex neurogenic bladder (i.e., urgency, frequency, urinary leakage, urge incontinence, dysuria).
Contraindications
Oxybutynin chloride is contraindicated in patients with untreated angle closure glaucoma and in patients with untreated narrow anterior chamber angles since anticholinergic drugs may aggravate these conditions. It is also contraindicated in partial or complete obstruction of the gastrointestinal tract, paralytic ileus, intestinal atony of the elderly or debilitated patient, megacolon, toxic megacolon complicating ulcerative colitis, severe colitis and myasthenia gravis. It is contraindicated in patients with obstructive uropathy and in patients with unstable cardiovascular status in acute hemorrhage.
Oxybutynin chloride is contraindicated in patients who have demonstrated hypersensitivity to the product.
Warnings
Oxybutynin chloride, when administered in the presence of high environmental temperature, can cause heat prostration (fever and heat stroke due to decreased sweating). Diarrhea may be an early symptom of incomplete intestinal obstruction, especially in patients with ileostomy or colostomy. In this instance, treatment with oxybutynin chloride would be inappropriate and possibly harmful.
Oxybutynin chloride may produce drowsiness or blurred vision. The patient should be cautioned regarding activities requiring mental alertness such as operating a motor vehicle or other machinery or performing hazardous work while taking this drug.
Alcohol or other sedative drugs may enhance the drowsiness caused by oxybutynin.
Precautions
Oxybutynin chloride should be used with caution in the elderly and in all patients with autonomic neuropathy, hepatic or renal disease. Oxybutynin may aggravate the symptoms of hyperthyroidism, coronary heart disease, congestive heart failure, cardiac arrhythmias, hiatal hernia, tachycardia, hypertension, and prostatic hypertrophy.
Administration of oxybutynin chloride to patients with ulcerative colitis may suppress intestinal motility to the point of producing a paralytic ileus and precipitate or aggravate toxic megacolon, a serious complication of the disease.
Carcinogenesis, Mutagenesis, Impairment of Fertility
A 24-month study in rats at dosages up to approximately 400 times the recommended human dosage showed no evidence of carcinogenicity.
Oxybutynin showed no increase in mutagenic activity when tested in Schizosac-charomyces pompholiciformis, Saccharomyces cerevisiae, and Salmonella typhimurium test symptoms. Reproduction studies in the hamster, rabbit, rat, and mouse have shown no definite evidence of impaired fertility.
Pregnancy
Category B: Reproduction studies in the hamster, rabbit, rat, and mouse have shown no definite evidence of impaired fertility or harm to the animal fetus. The safety of oxybutynin chloride administered to women who are or who may become pregnant has not been established. Therefore, oxybutynin should not be given to pregnant women unless, in the judgment of the physician, the probable clinical benefits outweigh the possible hazards.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when oxybutynin chloride is administered to a nursing woman.
Pediatric Use
The safety and efficacy of oxybutynin chloride administration have been demonstrated for children 5 years of age and older (see DOSAGE AND ADMINISTRATION). However, as there is insufficient clinical data for children under age 5, oxybutynin is not recommended for this age group.
Adverse Reactions
Following administration of oxybutynin chloride, the symptoms that can be associated with the use of other anticholinergic drugs may occur:
Cardiovascular: Palpitations, tachycardia, vasodilatation.
Dermatologic: Decreased sweating, rash.
Gastrointestinal/Genitourinary: Constipation, decreased gastrointestinal motility, dry mouth, nausea, urinary hesitance and retention.
Nervous System: Asthenia, dizziness, drowsiness, hallucinations, insomnia, restlessness.
Ophthalmic: Amblyopia, cycloplegia, decreased lacrimation, mydriasis.
Other: Impotence, suppression of lactation.
Overdosage
The symptoms of overdosage with oxybutynin chloride may be any of those seen with other anticholinergic agents. Symptoms may include signs of central nervous system excitation (e.g., restlessness, tremor, irritability, convulsions, delirium, hallucinations), flushing, fever, nausea, vomiting, tachycardia, hypotension or hypertension, respiratory failure, paralysis, or coma.
In the event of an overdose or exaggerated response, treatment should be symptomatic and supportive. Maintain respiration and induce emesis or perform gastric lavage (emesis is contraindicated in precomatose, convulsive, or psychotic state). Activated charcoal may be administered as well as a cathartic. Physostigmine may be considered to reverse symptoms of anticholinergic intoxication.
Hyperpyrexia may be treated symptomatically with ice bags or other cold applications and alcohol sponges.
Dosage And Administration
Adults: The usual dose is one 5 mg tablet two to three times a day. The maximum recommended dose is one tablet (5 mg) four times a day.
Children over 5 years of age: The usual dose is one 5 mg tablet two times a day. The maximum recommended dose is one tablet (5 mg) three times a day.
How Supplied
Oxybutynin Chloride Tablets, USP:
5 mg — Very pale blue, round, scored tablets.
Debossed: PLIVA 456
They are supplied by State of Florida DOH Central Pharmacy as follows:
NDC Strength Quantity/Form Color Source Prod. Code 53808-0747-1 5 mg 30 Tablets in a Buler Pack Very pale blue 50111-456
Dispense in a tight, light-resistant container as defined in the USP.
Store at 20°-25°C (68°-77°F) [See USP Controlled Room Temperature].
Manufactured by:
PLIVA®, Inc.
East Hanover, NJÂ 07936
This Product was Repackaged By:
State of Florida DOH Central Pharmacy 104-2 Hamilton Park Drive Tallahassee, FL 32304 United States
Label Image 5mg
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site