Phenobarbital (phenobarbital 60 mg) Dailymed
Generic: phenobarbital is used for the treatment of Airway Obstruction Seizures, Febrile Epilepsies, Partial Epilepsy, Tonic-Clonic Hyperbilirubinemia Porphyrias Pregnancy Liver Failure Epilepsy, Benign Neonatal
IMPRINT: WEST WARD 445
SHAPE: round
COLOR: white
All Imprints
phenobarbital 15 mg oral tablet - westward 445 round white
phenobarbital 100 mg - ww 458 round white
phenobarbital 30 mg - west ward 450 round white
phenobarbital 15 mg - west ward 445 round white
phenobarbital 60 mg - ww 455 round white
Go PRO for all pill images
Description
Phenobarbital is a barbituric acid derivative for oral administration and occurs as a white, odorless, slightly bitter powder that is soluble in chloroform, freely soluble in alcohol or ether, and slightly soluble in water. Its saturated solution has a pH of about 5.6. Chemically, it is 5-ethyl-5-phenylbarbituric acid with the molecular formula C12H12N2O3 (232.24). The structural formula is as follows:
Each Phenobarbital Tablet, USP contains 15 mg, 30 mg, 60 mg or 100 mg of phenobarbital, USP.
Inactive Ingredients are as follows:
Anhydrous Lactose, Colloidal Silicon Dioxide, Corn Starch, Docusate Sodium with Sodium Benzoate, Lactose Monohydrate, Magnesium Stearate, Microcrystalline Cellulose, and Sodium Starch Glycolate.
Clinical Pharmacology
Phenobarbital, a long-acting barbiturate, is a central nervous system depressant. In ordinary doses, the drug acts as a sedative and anticonvulsant. Its onset of action occurs within 30 minutes, and the duration of action ranges from 5 to 6 hours. It is detoxified in the liver.
Indications And Usage
Phenobarbital Tablets, USP are indicated for use as a sedative or anticonvulsant.
Contraindications
Phenobarbital is contraindicated in patients who are hypersensitive to barbiturates. In such patients, severe hepatic damage can occur from ordinary doses and is usually associated with dermatitis and involvement of parenchymatous organs. A personal or familial history of acute intermittent porphyria represents one of the few absolute contraindications to the use of barbiturates. Phenobarbital is also contraindicated in patients with marked impairment of liver function, or respiratory disease in which dyspnea or obstruction is evident. It should not be administered to persons with known previous addiction to the sedative/hypnotic group, since ordinary doses may be ineffectual and may contribute to further addiction.
Warnings
In small doses, the barbiturates may increase the reaction to painful stimuli. Taken by themselves, the barbiturates cannot be relied upon to relieve pain or even to produce sedation or sleep in the presence of severe pain.
Precautions
General Precautions
Barbiturates induce liver microsomal enzyme activity. This accelerates the biotransformation of various drugs and is probably part of the mechanism of the tolerance encountered with barbiturates. Phenobarbital, therefore, should be used with caution in patients with decreased liver function. This drug should also be administered cautiously to patients with a history of drug dependence or abuse (see DRUG ABUSE AND DEPENDENCE ).
Phenobarbital may decrease the potency of coumarin anticoagulants; therefore, patients receiving such concomitant therapy should have more frequent prothrombin determinations. As with other sedatives and hypnotics, elderly or debilitated patients may react to barbiturates with marked exclient, depression, or confusion.
The systemic effects of exogenous hydrocortisone and endogenous hydrocortisone (cortisol) may be diminished by phenobarbital. Thus, this product should be administered with caution to patients with borderline hypoadrenal function, regardless of whether it is of pituitary or of primary adrenal origin.
Information for Patients
Phenobarbital may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks, such as driving a car or operating machinery. The patient should be cautioned accordingly.
Drug Interactions
Phenobarbital in combination with alcohol, tranquilizers, and other central nervous system depressants has additive depressant effects, and the patient should be so advised. Patients taking this drug should be warned not to exceed the dosage recommended by their physician. Toxic effects and fatalities have occurred following overdoses of phenobarbital alone and in combination with other central nervous system depressants. Caution should be exercised in prescribing unnecessarily large amounts of phenobarbital for patients who have a history of emotional disturbances or suicidal ideation or who have misused alcohol and other CNS drugs (see OVERDOSAGE ).
Usage in Pregnancy
Pregnancy Category B: Reproduction studies have been performed in animals and have revealed no evidence of impaired fertility or harm to the fetus due to phenobarbital. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
Caution should be exercised when phenobarbital is administered to a nursing woman.
Adverse Reactions
The following adverse reactions have been reported:
CNS Depression
Residual sedation or ‚Äúhangover‚ÄĚ, drowsiness, lethargy, and vertigo. Emotional disturbances and phobias may be accentuated. In some persons, barbiturates such as phenobarbital repeatedly produce exclient rather than depression, and the patient may appear to be inebriated. Like other nonanalgesic hypnotic drugs, barbiturates, such as phenobarbital, when given in the presence of pain, may cause restlessness, exclient, and even delirium. Rarely, the use of barbiturates results in localized or diffused myalgic, neuralgic, or arthritic pain, especially in psychoneurotic patients with insomnia. The pain may appear in paroxysms, is most intense in the early morning hours, and is most frequently located in the region of the neck, shoulder girdle, and upper limbs. Symptoms may last for days after the drug is discontinued.
Respiratory/Circulatory
Respiratory depression, apnea, circulatory collapse.
Allergic
Acquired hypersensitivity to barbiturates consists chiefly in allergic reactions that occur especially in persons who tend to have asthma, urticaria, angioedema, and similar conditions. Hypersensitivity reactions in this category include localized swelling, particularly of the eyelids, cheeks, or lips, and erythematous dermatitis. Rarely, exfoliative dermatitis (e.g., Stevens-Johnson syndrome and toxic epidermal necrolysis) may be caused by phenobarbital and can prove fatal. The skin eruption may be associated with fever, delirium, and marked degenerative changes in the liver and other parenchymatous organs. In a few cases, megaloblastic anemia has been associated with the chronic use of phenobarbital.
Other
Nausea and vomiting; headache.
To report SUSPECTED ADVERSE REACTIONS, contact Hikma Pharmaceuticals USA Inc. at 1-800-962-8364, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Abuse And Dependence
Controlled Substance: Phenobarbital is a Schedule IV drug.
Dependence
Prolonged, uninterrupted use of barbiturates (particularly the short-acting drugs), even in therapeutic doses, may result in psychic and physical dependence. Withdrawal symptoms due to physical dependence following chronic use of large doses of barbiturates may include delirium, convulsions, and death.
Overdosage
The signs and symptoms of barbiturate poisoning are referable especially to the central nervous system and the cardiovascular system. Moderate intoxication resembles alcoholic inebriation. In severe intoxication, the patient is comatose, the level of reflex activity conforming in a general way to the intensity of the central depression. The deep reflexes may persist for some time despite coexistent coma. The Babinski sign is often positive. The EEG may be of the ‚Äúburst-suppression‚ÄĚ type, with brief periods of electrical silence. The pupils may be constricted and react to light, but late in the course of barbiturate poisoning they may show hypoxic paralytic dilatation. Respiration is affected early. Breathing may be either slow or rapid and shallow; Cheyne-Stokes rhythm may be present. Respiratory minute volume is diminished, and hypoxia and respiratory acidosis may develop. The blood pressure falls, owing partly to depression of medullary vasomotor centers; partly to a direct action of the drug on the myocardium, sympathetic ganglia, and vascular smooth muscle; partly to hypoxia.
The patient thus develops a typical shock syndrome, with a weak and rapid pulse, cold and clammy skin, and a rise in the hematocrit. Respiratory complications (atelectasis, pulmonary edema, and bronchopneumonia) and renal failure are much dreaded and not infrequent concomitant of severe barbiturate poisoning. There is usually hypothermia, sometimes with temperatures as low as 32¬įC.
Treatment
General management should consist of symptomatic and supportive therapy, including gastric lavage, administration of intravenous fluids, and maintenance of blood pressure, body temperature and adequate respiratory exchange. Dialysis will increase the rate of removal of barbiturates from the body fluids. Antibiotics may be required to control pulmonary complications.
Dosage And Administration
Oral Sedative Dose
Adults: 30 to 120 mg daily in 2 or 3 divided doses.
Children: 6 mg/kg of body weight daily in 3 divided doses.
Oral Hypnotic Dose
Adults: 100 to 320 mg.
Oral Anticonvulsant Dose,
Adults: 50 to 100 mg 2 or 3 times daily.
Children: 15 to 50 mg 2 or 3 times daily.
How Supplied
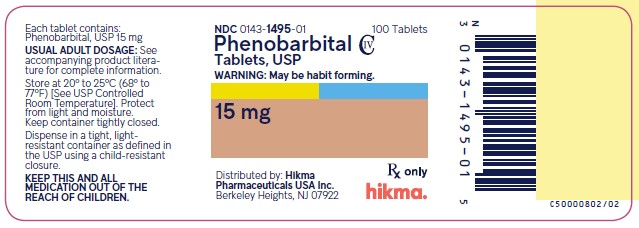
Phenobarbital Tablets USP, 15 mg
White, Round Tablet; Debossed ‚ÄúWW 445‚ÄĚ on one side and plain on the other side.¬† ¬† ¬† ¬† ¬† ¬† ¬†¬†
              NDC 0143-1495-01: Bottle of 100 tablets                NDC 0143-1495-05: Bottle of 500 tablets  
Phenobarbital Tablets USP, 30 mg
White, Round, Scored Tablet; Debossed ‚ÄúWW 450‚ÄĚ on one side and Scored on the other side.
             NDC 0143-1500-01: Bottle of 100 tablets                 NDC 0143-1500-05: Bottle of 500 tablets  
Phenobarbital Tablets USP, 60 mg
White, Round Tablet; Debossed ‚ÄúWW 455‚ÄĚ on one side and plain on the other side.
             NDC 0143-1455-01: Bottle of 100 tablets               NDC 0143-1455-05: Bottle of 500 tablets
Phenobarbital Tablets USP, 100 mg
White, Round, Scored Tablet; Debossed ‚ÄúWW 458‚ÄĚ on one side and Scored on the other side.
             NDC 0143-1458-01: Bottle of 100 tablets                NDC 0143-1458-05: Bottle of 500 tablets
Store at 20¬į to 25¬įC (68¬į to 77¬įF) [See USP Controlled Room Temperature]. Protect from light and moisture.
Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
Distributed by: Hikma Pharmaceuticals USA Inc. Berkeley Heights,  NJ 07922
C50000803/02      Revised August 2022
Principal Display Panel
NDC 0143-1495-01 Phenobarbital Tablets USP  15 mg 100 Tablets Rx Only

Principal Display Panel
NDC 0143-1500-01 Phenobarbital Tablets USP  30 mg 100 Tablets Rx Only

Principal Display Panel
NDC 0143-1455-01 Phenobarbital Tablets USP  60 mg 100 Tablets Rx Only
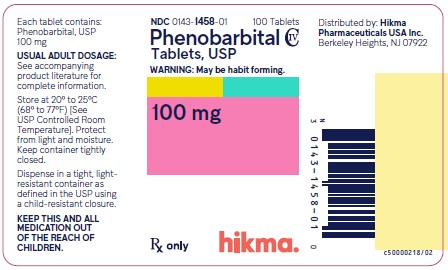
Principal Display Panel
NDC 0143-1458-01 Phenobarbital Tablets USP  100 mg 100 Tablets Rx Only

Package Label.principal Display Panel
Package Label.principal Display Panel
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site


