Propoxyphene and Acetaminophen (propoxyphene napsylate 100 mg acetaminophen 650 mg) Dailymed
Generic: propoxyphene napsylate and acetaminophen is used for the treatment of Fever Glucosephosphate Dehydrogenase Deficiency Liver Diseases Pain Asthma Hypercapnia Intestinal Pseudo-Obstruction Pregnancy Respiratory Insufficiency
IMPRINT: 5114 V
SHAPE: oval
COLOR: orange
All Imprints
propoxyphene napsylate 100 mg acetaminophen 650 mg - 5114 v oval pink
propoxyphene and acetaminophen (propoxyphene napsylate and acetaminophen) tablet, film coated - 5114 v oval orange
propoxyphene and acetaminophen (propoxyphene napsylate and acetaminophen) tablet, film coated - 5114 v oval pink
propoxyphene and acetaminophen (propoxyphene napsylate and acetaminophen) tablet, film coated - 5113 v oval white
Boxed Warning
Warnings
- There have been numerous cases of accidental and intentional overdose with propoxyphene products either alone or in combination with other CNS depressants, including alcohol. Fatalities within the first hour of overdosage are not uncommon. Many of the propoxyphene-related deaths have occurred in patients with previous histories of emotional disturbances or suicidal ideation/attempts and/or concomitant administration of sedatives, tranquilizers, muscle relaxants, antidepressants, or other CNS-depressant drugs. Do not prescribe propoxyphene for patients who are suicidal or have a history of suicidal ideation.
- The metabolism of propoxyphene may be altered by strong CYP3A4 inhibitors (such as ritonavir, ketoconazole, itraconazole, troleandomycin, clarithromycin, nelfinavir, nefazadone, amiodarone, amprenavir, aprepitant, diltiazem, erythromycin, fluconazole, fosamprenavir, grapefruit juice, and verapamil) leading to enhanced propoxyphene plasma levels. Patients receiving propoxyphene and any CYP3A4 inhibitor should be carefully monitored for an extended period of time and dosage adjustments should be made if warranted (see CLINICAL PHARMACOLOGY – Drug Interactions , and WARNINGS , PRECAUTIONS and DOSAGE AND ADMINISTRATION for further information).
Boxed Warning
Warnings
Risk of Overdose
Respiratory Depression
Hypotensive Effect
Head Injury and Increased Intracranial Pressure
Drug Interactions
Usage in Ambulatory Patients
Use with Other Acetaminophen-Containing Agents
Use with Alcohol
Go PRO for all pill images
Warnings
- There have been numerous cases of accidental and intentional overdose with propoxyphene products either alone or in combination with other CNS depressants, including alcohol. Fatalities within the first hour of overdosage are not uncommon. Many of the propoxyphene-related deaths have occurred in patients with previous histories of emotional disturbances or suicidal ideation/attempts and/or concomitant administration of sedatives, tranquilizers, muscle relaxants, antidepressants, or other CNS-depressant drugs. Do not prescribe propoxyphene for patients who are suicidal or have a history of suicidal ideation.
- The metabolism of propoxyphene may be altered by strong CYP3A4 inhibitors (such as ritonavir, ketoconazole, itraconazole, troleandomycin, clarithromycin, nelfinavir, nefazadone, amiodarone, amprenavir, aprepitant, diltiazem, erythromycin, fluconazole, fosamprenavir, grapefruit juice, and verapamil) leading to enhanced propoxyphene plasma levels. Patients receiving propoxyphene and any CYP3A4 inhibitor should be carefully monitored for an extended period of time and dosage adjustments should be made if warranted (see CLINICAL PHARMACOLOGY – Drug Interactions , and WARNINGS , PRECAUTIONS and DOSAGE AND ADMINISTRATION for further information).
Description
Propoxyphene Napsylate, USP is an odorless, white crystalline powder with a bitter taste. It is very slightly soluble in water and soluble in methanol, ethanol, chloroform, and acetone. Chemically, it is (αS,1R)-α-[2-(Dimethylamino)-1-methylethyl]-α-phenylphenethyl propionate compound with 2-napthalenesulfonic acid (1:1) monohydrate, which can be represented by the accompanying structural formula.
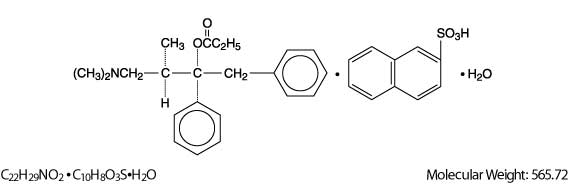
Propoxyphene napsylate differs from propoxyphene hydrochloride in that it allows more stable liquid dosage forms and tablet formulations. Because of differences in molecular weight, a dose of 100 mg (176.8 ÎĽmol) of propoxyphene napsylate is required to supply an amount of propoxyphene equivalent to that present in 65 mg (172.9 ÎĽmol) of propoxyphene hydrochloride.
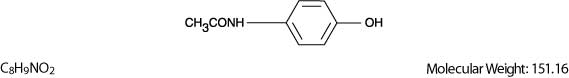
Acetaminophen, 4'-hydroxyacetanilide, is a non-opiate, non-salicylate analgesic and antipyretic which occurs as a white, odorless, crystalline powder, possessing a slightly bitter taste. The molecular formula for acetaminophen is C8H9NO2 and the molecular weight is 151.16. It may be represented by the following structural formula.
Propoxyphene Napsylate and Acetaminophen Tablets, USP 50 mg/325 mg Each tablet contains:Â Â Â Â Â Â Â Propoxyphene Napsylate ............................50 mg (88.4 ÎĽmol)Â Â Â Â Â Â Â Acetaminophen .........................................325 mg (2150 ÎĽmol)Â In addition each tablet contains the following inactive ingredients: carnauba wax, D&C Yellow #10 Aluminum Lake, FD&C Red #40 Aluminum Lake, hypromellose, lactose monohydrate, magnesium stearate, polyethylene glycol, polysorbate 80, sodium starch glycolate and titanium dioxide.
Propoxyphene Napsylate and Acetaminophen Tablets, USP 100 mg/650 mg Each tablet contains:Â Â Â Â Â Â Â Propoxyphene Napsylate ...........................100 mg (176.8 ÎĽmol)Â Â Â Â Â Â Â Acetaminophen ..........................................650 mg (4300 ÎĽmol)In addition each tablet contains the following inactive ingredients: carnauba wax, hypromellose, lactose monohydrate, magnesium stearate, polyethylene glycol, polysorbate 80, sodium starch glycolate and titanium dioxide. The orange tablets also contain D&C Yellow #10 Aluminum Lake and FD&C Red #40 Aluminum Lake. The pink tablets also contain FD&C Red #40 Aluminum Lake.
Clinical Pharmacology
Pharmacology
Propoxyphene is a centrally acting opiate analgesic. In vitro studies demonstrated propoxyphene and the metabolite norpropoxyphene inhibit sodium channels (local anesthetic effect) with norpropoxyphene being approximately 2-fold more potent than propoxyphene and propoxyphene approximately 10-fold more potent than lidocaine. Propoxyphene and norpropoxyphene inhibit the voltage-gated potassium current carried by cardiac rapidly activating delayed rectifier (hERG) channels with approximately equal potency. It is unclear if the effects on ion channels occur within therapeutic dose range.
Acetaminophen is a non-opiate, non-salicylate analgesic and antipyretic. The site and mechanism for the analgesic effect of acetaminophen has not been determined. The antipyretic effect of acetaminophen is mediated through activity in the hypothalamic heat-regulating centers. Acetaminophen inhibits prostaglandin synthetase. Therapeutic doses of acetaminophen have negligible effects on the cardiovascular or respiratory systems; however, toxic doses may cause circulatory failure and rapid, shallow breathing.
Pharmacokinetics
Absorption
Peak plasma concentrations of propoxyphene are reached in 2 to 2.5 h. After a 65-mg oral dose of propoxyphene hydrochloride, peak plasma levels of 0.05 to 0.1 ÎĽg/mL for propoxyphene and 0.1 to 0.2 ÎĽg/mL for norpropoxyphene (major metabolite) are achieved. Repeated doses of propoxyphene at 6 h intervals lead to increasing plasma concentrations, with a plateau after the ninth dose at 48 h. Propoxyphene has a half-life of 6 to 12 h, whereas that of norpropoxyphene is 30 to 36 h.
Acetaminophen is absorbed from the gastrointestinal tract and has a plasma half-life of 1.25 to 3 h, which may be increased by liver damage and following overdosage.
Distribution
Propoxyphene is about 80% bound to proteins and has a large volume of distribution, 16 L/kg.
Acetaminophen is relatively uniformly distributed throughout most body fluids. Binding of the drug to plasma proteins is variable; only 20% to 50% may be bound at the concentrations encountered during acute intoxication.
Metabolism
Propoxyphene undergoes extensive first-pass metabolism by intestinal and hepatic enzymes. The major route of metabolism is cytochrome CYP3A4 mediated N-demethylation to norpropoxyphene, which is excreted by the kidneys. Ring hydroxylation and glucuronide formation are minor metabolic pathways.
Acetaminophen is extensively metabolized in the liver. Less than 5% of acetaminophen dose is excreted unchanged in the kidney. About 85% of an acetaminophen dose is metabolized by conjugation, mainly glucuronidation via UDP-glucuronosyltransferase (mainly UGT1A6) and to a lesser extent sulfation via sulfotransferase (mainly SLT1A1 and SLT1A3). The glucuronide and sulfate conjugates are nontoxic and are largely excreted in the urine and bile. About 8-10% of an acetaminophen dose is oxidized by cytochrome CYP2E1 to form the toxic reactive intermediate, N-acetyl-p-benzoquinone imine (NAPQI). NAPQI is further metabolized via glutathione (GSH) conjugation, yielding non-toxic thiol metabolites including cysteine, mercapturate, methylthioacetaminophen, and methanesulfinylacetaminophen that are excreted in the urine. Acetaminophen is also oxidized at a low percentage by cytochrome CYP2A6 to form inert catechols (e.g., methoxyacetaminophen).
Excretion
In 48 h, approximately 20 to 25% of the administered dose of propoxyphene is excreted via the urine, most of which is free or conjugated norpropoxyphene. The renal clearance rate of propoxyphene is 2.6 L/min.
Elimination of acetaminophen is principally by liver metabolism (conjugation) and subsequent renal excretion of metabolites. Approximately 85% of an oral dose appears in the urine within 24 hours of administration, most as the glucuronide conjugate, with small amounts of other conjugates and unchanged drug.
Special Populations
Geriatric Patients
After oral administration of propoxyphene in elderly patients (70-78 years), much longer half-lives of propoxyphene and norpropoxyphene have been reported (propropoxyphene 13 to 35 h, norpropoxyphene 22 to 41 h). In addition, the AUC was an average of 3-fold higher and the Cmax was an average of 2.5-fold higher in the elderly when compared to a younger (20-28 years) population. Longer dosage intervals may be considered in the elderly because the metabolism of propoxyphene may be reduced in this patient population. After multiple oral doses of propoxyphene in elderly patients (70-78 years), the Cmax of the metabolite (norpropoxyphene) was increased 5-fold.
Pediatric Patients
Neither propoxyphene alone nor in combination with acetaminophen has been studied in pediatric patients.
Hepatic Impairment
No formal pharmacokinetic study of either propoxyphene alone or in combination with acetaminophen has been conducted in patients with mild, moderate or severe hepatic impairment.
After oral administration of propoxyphene in patients with cirrhosis, plasma concentrations of propoxyphene were considerably higher and norpropoxyphene concentrations were much lower than in control patients. This is presumably because of a decreased first-pass metabolism of orally administered propoxyphene in these patients. The AUC ratio of norpropoxyphene: propoxyphene was significantly lower in patients with cirrhosis (0.5 to 0.9) than in controls (2.5 to 4).
Compared to healthy subjects, acetaminophen had a lower total clearance and longer half-life in patients with liver disease. Decreased metabolite formation clearance (8-42%) was observed in subjects with liver disease compared to healthy subjects after both single and multiple-doses (at steady state). In addition, there is an increase in the amount of acetaminophen excreted unchanged in the urine (4.7% vs. 2.5%) in patients with liver disease compared to healthy subjects after repeat doses, suggesting that more acetaminophen was excreted by renal elimination in the liver disease state.
Renal Impairment
No formal pharmacokinetic study of either propoxyphene alone or in combination with acetaminophen has been conducted in patients with mild, moderate or severe renal impairment.
After oral administration of propoxyphene in anephric patients, the AUC and Cmax values were an average of 76% and 88% greater, respectively. Dialysis removes only insignificant amounts (8%) of administered dose of propoxyphene.
Drug Interactions
The metabolism of propoxyphene may be altered by strong CYP3A4 inhibitors (such as ritonavir, ketoconazole, itraconazole, troleandomycin, clarithromycin, nelfinavir, nefazadone, amiodarone, amprenavir, aprepitant, diltiazem, erythromycin, fluconazole, fosamprenavir, grapefruit juice, and verapamil) leading to enhanced propoxyphene plasma levels. On the other hand, strong CYP3A4 inducers such as rifampin may lead to enhanced metabolite (norpropoxyphene) levels.
Propoxyphene is also thought to possess CYP3A4 and CYP2D6 enzyme inhibiting properties. Coadministration with a drug that is a substrate of CYP3A4 or CYP2D6, may result in higher plasma concentrations and increased pharmacologic or adverse effects of that drug.
Clinical Studies
The efficacy of propoxyphene in combination with acetaminophen was studied in seven single-dose, randomized, double-blind, placebo-controlled trials in patients with mild to severe postpartum pain. One of the studies demonstrated that both propoxyphene and acetaminophen in the combination contributed to a greater reduction in pain than acetaminophen and propoxyphene alone and that propoxyphene was superior to placebo.
There is insufficient information available to assess efficacy of propoxyphene in combination with acetaminophen in patients with chronic pain.
Indication
Propoxyphene napsylate and acetaminophen tablets, USPÂ are indicated for the relief of mild to moderate pain.
Contraindications
Propoxyphene napsylate and acetaminophen tablets are contraindicated in patients with known hypersensitivity to propoxyphene or acetaminophen.
Propoxyphene napsylate and acetaminophen tablets are contraindicated in patients with significant respiratory depression (in unmonitored settings or the absence of resuscitative equipment) and patients with acute or severe asthma or hypercarbia.
Propoxyphene napsylate and acetaminophen tablets are contraindicated in any patient who has or is suspected of having paralytic ileus.
Warnings
Risk of Overdose
There have been numerous cases of accidental and intentional overdose with propoxyphene products either alone or in combination with other CNS depressants, including alcohol. Fatalities within the first hour of overdosage are not uncommon. Many of the propoxyphene-related deaths have occurred in patients with previous histories of emotional disturbances or suicidal ideation/attempts and/or concomitant administration of sedatives, tranquilizers, muscle relaxants, antidepressants, or other CNS-depressant drugs. Do not prescribe propoxyphene for patients who are suicidal or have a history of suicidal ideation.
Respiratory Depression
Respiratory depression is the chief hazard from all opioid agonist preparations. Respiratory depression occurs most frequently in elderly or debilitated patients, usually following large initial doses in non-tolerant patients, or when opioids are given in conjunction with other agents that depress respiration. Propoxyphene napsylate and acetaminophen should be used with extreme caution in patients with significant chronic obstructive pulmonary disease or cor pulmonale, and in patients having substantially decreased respiratory reserve, hypoxia, hypercapnia, or pre-existing respiratory depression. In such patients, even usual therapeutic doses of propoxyphene napsylate and acetaminophen may decrease respiratory drive to the point of apnea. In these patients alternative non-opioid analgesics should be considered, and opioids should be employed only under careful medical supervision at the lowest effective dose.
Hypotensive Effect
Propoxyphene napsylate and acetaminophen, like all opioid analgesics, may cause severe hypotension in an individual whose ability to maintain blood pressure has been compromised by a depleted blood volume, or after concurrent administration with drugs such as phenothiazines or other agents which compromise vasomotor tone. Propoxyphene napsylate and acetaminophen may produce orthostatic hypotension in ambulatory patients. Propoxyphene napsylate and acetaminophen, like all opioid analgesics, should be administered with caution to patients in circulatory shock, since vasodilatation produced by the drug may further reduce cardiac output and blood pressure.
Head Injury and Increased Intracranial Pressure
The respiratory depressant effects of narcotics and their capacity to elevate cerebrospinal fluid pressure may be markedly exaggerated in the presence of head injury, other intracranial lesions or a pre-existing increase in intracranial pressure. Furthermore, narcotics produce adverse reactions which may obscure the clinical course of patients with head injuries.
Drug Interactions
The concomitant use of propoxyphene and CNS depressants, including alcohol, can result in potentially serious adverse events including death. Because of its added depressant effects, propoxyphene should be prescribed with caution for those patients whose medical condition requires the concomitant administration of sedatives, tranquilizers, muscle relaxants, antidepressants, or other CNS-depressant drugs.
Usage in Ambulatory Patients
Propoxyphene may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks, such as driving a car or operating machinery. The patient should be cautioned accordingly.
Use with Other Acetaminophen-Containing Agents
Due to the potential for acetaminophen hepatotoxicity at doses higher than the recommended dose, propoxyphene napsylate and acetaminophen should not be used concomitantly with other acetaminophen-containing products.
Use with Alcohol
Hepatotoxicity and severe hepatic failure occurred in chronic alcoholics following therapeutic doses of acetaminophen. Patients should be cautioned about the concomitant use of propoxyphene products and alcohol because of potentially serious CNS-additive effects of these agents that can lead to death.
Precautions
Tolerance and Physical Dependence
Tolerance is the need for increasing doses of opioids to maintain a defined effect such as analgesia (in the absence of disease progression or other external factors). Physical dependence is manifested by withdrawal symptoms after abrupt discontinuation of a drug or upon administration of an antagonist. Physical dependence and tolerance are not unusual during chronic opioid therapy.
The opioid abstinence or withdrawal syndrome is characterized by some or all of the following: restlessness, lacrimation, rhinorrhea, yawning, perspiration, chills, myalgia, and mydriasis. Other symptoms also may develop, including: irritability, anxiety, backache, joint pain, weakness, abdominal cramps, insomnia, nausea, anorexia, vomiting, diarrhea, or increased blood pressure, respiratory rate, or heart rate. In general, opioids should not be abruptly discontinued (see DOSAGE AND ADMINISTRATION: Cessation of Therapy ).
If propoxyphene napsylate and acetaminophen is abruptly discontinued in a physically dependent patient, an abstinence syndrome may occur (see DRUG ABUSE AND DEPENDENCE ). If signs and symptoms of withdrawal occur, patients should be treated by reinstitution of opioid therapy followed by gradual tapered dose reduction of propoxyphene napsylate and acetaminophen combined with symptomatic support (see DOSAGE AND ADMINISTRATION: Cessation of Therapy ).
Use in Pancreatic/Biliary Tract Disease
Propoxyphene napsylate and acetaminophen may cause spasm of the sphincter of Oddi and should be used with caution in patients with biliary tract disease, including acute pancreatitis. Opioids like propoxyphene napsylate and acetaminophen may cause increases in the serum amylase level.
Hepatic or Renal Impairment
Insufficient information exists to make appropriate dosing recommendations regarding the use of either propoxyphene alone or in combination with acetaminophen in patients with hepatic or renal impairment as a function of degree of impairment. Higher plasma concentrations and/or delayed elimination may occur in case of impaired hepatic function and/or impaired renal function (see CLINICAL PHARMACOLOGY ). If the drug is used in these patients, it should be used with caution because of the hepatic metabolism of propoxyphene and acetaminophen and renal excretion of their metabolites.
Information for Patients/Caregivers
- Patients should be advised to report pain and adverse experiences occurring during therapy. Individualization of dosage is essential to make optimal use of this medication.
- Patients should be advised not to adjust the dose of propoxyphene napsylate and acetaminophen tablets without consulting the prescribing professional.
- Patients should be advised that propoxyphene napsylate and acetaminophen may impair mental and/or physical ability required for the performance of potentially hazardous tasks (e.g., driving, operating heavy machinery).
- Patients should not combine propoxyphene napsylate and acetaminophen with central nervous system depressants (e.g., sleep aids, tranquilizers) except by the orders of the prescribing physician, because additive effects may occur.
- Patients should be instructed not to consume alcoholic beverages, including prescription and over-the-counter medications that contain alcohol, while using propoxyphene napsylate and acetaminophen tablets because of risk of serious adverse events including death.
- Women of childbearing potential who become, or are planning to become, pregnant should be advised to consult their physician regarding the effects of analgesics and other drug use during pregnancy on themselves and their unborn child.
- Patients should be advised that propoxyphene napsylate and acetaminophen is a potential drug of abuse. They should protect it from theft, and it should never be given to anyone other than the individual for whom it was prescribed.
- Patients should be advised that if they have been receiving treatment with propoxyphene napsylate and acetaminophen for more than a few weeks and cessation of therapy is indicated, it may be appropriate to taper the propoxyphene napsylate and acetaminophen dose, rather than abruptly discontinue it, due to the risk of precipitating withdrawal symptoms. Their physician can provide a dose schedule to accomplish a gradual discontinuation of the medication.
- Instruct patients not to consume any other medication that contain acetaminophen, including acetaminophen-based over-the-counter medications, while taking propoxyphene napsylate and acetaminophen tablets.
Drug Interactions with Propoxyphene
Propoxyphene is metabolized mainly via the human cytochrome P450 3A4 isoenzyme system (CYP3A4), therefore potential interactions may occur when propoxyphene is administered concurrently with agents that affect CYP3A4 activity.
The metabolism of propoxyphene may be altered by strong CYP3A4 inhibitors (such as ritonavir, ketoconazole, itraconazole, troleandomycin, clarithromycin, nelfinavir, nefazadone, amiodarone, amprenavir, aprepitant, diltiazem, erythromycin, fluconazole, fosamprenavir, grapefruit juice, and verapamil) leading to enhanced propoxyphene plasma levels. Coadministration with agents that induce CYP3A4 activity may reduce the efficacy of propoxyphene. Strong CYP3A4 inducers such as rifampin may lead to enhanced metabolite (norpropoxyphene) levels.
Propoxyphene is also thought to possess CYP3A4 and CYP2D6 enzyme inhibiting properties and coadministration with drugs that rely on either of these enzymes for metabolism may result in increased pharmacologic or adverse effects of that drug. Severe neurologic signs, including coma, have occurred with concurrent use of carbamazepine (metabolized by CYP3A4).
Increased risk of bleeding has been observed with warfarin-like agents when given along with propoxyphene; however, the mechanistic basis of this interaction is unknown.
CNS Depressants Patients receiving narcotic analgesics, general anesthetics, phenothiazines, other tranquilizers, sedative-hypnotics or other CNS depressants (including alcohol) concomitantly with propoxyphene napsylate and acetaminophen may exhibit an additive CNS depression. Interactive effects resulting in respiratory depression, hypotension, profound sedation, or coma may result if these drugs are taken in combination with the usual dosage of propoxyphene napsylate and acetaminophen. When such combined therapy is contemplated, the dose of one or both agents should be reduced.
Mixed Agonist/Antagonist Opioid Analgesics Agonist/antagonist analgesics (i.e., pentazocine, nalbuphine, butorphanol and buprenorphine) should be administered with caution to patients who have received or are receiving a course of therapy with a pure opioid agonist analgesic such as propoxyphene napsylate and acetaminophen. In this situation, mixed agonist/antagonist analgesics may reduce the analgesic effect of propoxyphene napsylate and acetaminophen and/or may precipitate withdrawal symptoms in these patients.
Monoamine Oxidase Inhibitors (MAOIs) MAOIs have been reported to intensify the effects of at least one opioid drug causing anxiety, confusion and significant depression of respiration or coma. The use of propoxyphene napsylate and acetaminophen is not recommended for patients taking MAOIs or within 14 days of stopping such treatment.
Drug Interactions with Acetaminophen
Alcohol: Hepatotoxicity has occurred in chronic alcoholics following various dose levels (moderate to excessive) of acetaminophen.
Anticholinergics: The onset of acetaminophen effect may be delayed or decreased slightly, but the ultimate pharmacological effect is not significantly affected by anticholinergics.
Oral Contraceptives: Increase in glucuronidation resulting in increased plasma clearance and a decreased half-life of acetaminophen.
Beta Blockers (Propranolol): Propranolol appears to inhibit the enzyme systems responsible for the glucuronidation and oxidation of acetaminophen. Therefore, the pharmacologic effects of acetaminophen may be increased.
Loop Diuretics: The effects of the loop diuretic may be decreased because acetaminophen may decrease renal prostaglandin excretion and decrease plasma renin activity.
Lamotrigine: Serum lamotrigine concentrations may be reduced, producing a decrease in therapeutic effects.
Probenecid: Probenecid may increase the therapeutic effectiveness of acetaminophen slightly.
Zidovudine: The pharmacologic effects of zidovudine may be decreased because of enhanced nonhepatic or renal clearance of zidovudine.
Carcinogenesis, Mutagenesis, Impairment of Fertility
The mutagenic and carcinogenic potential of propoxyphene and acetaminophen alone and in combination have not been evaluated.
In animal studies there was no effect of propoxyphene on mating behavior, fertility, duration of gestation, or parturition when rats were fed propoxyphene as a component of their daily diet at estimated daily propoxyphene intake up to 8-fold greater than the maximum human equivalent dose (HED) based on body surface area comparison. At this highest dose, fetal weight and survival on postnatal day 4 was reduced. Acetaminophen has not been studied in animals for effects on fertility and the effects on human fertility are unknown.
Pregnancy
Risk summary Pregnancy category C.There are no adequate and well-controlled studies of propoxyphene with acetaminophen in pregnant women. While there are limited data in the published literature, adequate animal reproduction studies have not been conducted with propoxyphene or acetaminophen. Therefore, it is not known whether propoxyphene or acetaminophen can affect reproduction or cause fetal harm when administered to a pregnant woman. Propoxyphene with acetaminophen should be given to a pregnant woman only if clearly needed.
Clinical considerations Acetaminophen, propoxyphene and its major metabolite, norpropoxyphene, cross the human placenta. Neonates whose mothers have taken opiates chronically may exhibit respiratory depression or withdrawal symptoms.
Data In published animal reproduction studies, no teratogenic effects occurred in offspring born to pregnant rats or rabbits that received propoxyphene during organogenesis. Pregnant animals received propoxyphene doses approximately 10-fold (rats) and 4-fold (rabbits) the maximum recommended human dose (based on mg/m2 body surface area comparison).
Nursing Mothers
Propoxyphene, norpropoxyphene (major metabolite), and acetaminophen are excreted in human milk. Published studies of nursing mothers using propoxyphene detected no adverse effects in nursing infants. Based on a study of six mother-infant pairs, an exclusively breastfed infant receives approximately 2% of the maternal weight-adjusted dose. Norpropoxyphene is renally excreted and renal clearance is lower in neonates than in adults. Therefore, it is possible that prolonged maternal propoxyphene use could result in norpropoxyphene accumulation in a breastfed infant. Watch breastfeeding infants for signs of sedation including poor feeding, somnolence, or respiratory depression. Caution should be exercised when propoxyphene napsylate and acetaminophen is administered to a nursing woman.
Pediatric Patients
Safety and effectiveness in pediatric patients have not been established.
Elderly Patients
Clinical studies of propoxyphene napsylate and acetaminophen did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. However, postmarketing reports suggest that patients over the age of 65 may be more susceptible to CNS-related side effects. Therefore, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. Decreased total daily dosage should be considered (see DOSAGE AND ADMINISTRATION ).
Adverse Reactions
During clinical trials, the most frequently reported adverse reactions were dizziness, sedation, nausea, and vomiting. Other adverse reactions include constipation, abdominal pain, skin rashes, lightheadedness, headache, weakness, euphoria, dysphoria, hallucinations, and minor visual disturbances.
The most frequently reported postmarketing adverse events have included completed suicide, accidental and intentional overdose, drug dependence, cardiac arrest, coma, drug ineffective, drug toxicity, nausea, respiratory arrest, cardio-respiratory arrest, death, vomiting, dizziness, convulsion, confusional state, and diarrhea.
Additional adverse experiences reported through postmarketing surveillance include:
Cardiac disorders: arrhythmia, bradycardia, cardiac/respiratory arrest, congestive arrest, congestive heart failure (CHF), tachycardia, myocardial infarction (MI)
Eye disorder: eye swelling, vision blurred
General disorder and administration site conditions: drug ineffective, drug interaction, drug tolerance, influenza type illness, drug withdrawal syndrome
Gastrointestinal disorder: gastrointestinal bleed, acute pancreatitis
Hepatobiliary disorder: hepatic steatosis, hepatomegaly, hepatocellular injury
Immune system disorder: hypersensitivity
Injury poisoning and procedural complications: drug toxicity, hip fracture, multiple drug overdose, narcotic overdose
Investigations: blood pressure decreased, heart rate elevated/abnormal
Metabolism and nutrition disorder: metabolic acidosis
Nervous system disorder: ataxia, coma, dizziness, somnolence, syncope
Psychiatric: abnormal behavior, confusional state, hallucinations, mental status change
Respiratory, thoracic, and mediastinal disorders: respiratory depression, dyspnoea
Skin and subcutaneous tissue disorder: rash, itch
Liver dysfunction has been reported in association with both active components of propoxyphene napsylate and acetaminophen tablets. Propoxyphene therapy has been associated with abnormal liver function tests and, more rarely, with instances of reversible jaundice (including cholestatic jaundice). Hepatic necrosis may result from acute overdose of acetaminophen (see OVERDOSAGE ). In chronic ethanol abusers, this has been reported rarely with short-term use of acetaminophen dosages of 2.5 to 10 g/day. Fatalities have occurred.
There have also been postmarketing reports of renal papillary necrosis associated with chronic acetaminophen use, particularly when the dosage is greater than recommended and when combined with aspirin. Subacute painful myopathy has been reported following chronic propoxyphene overdosage.
Drug Abuse And Dependence
Controlled Substance
Propoxyphene napsylate and acetaminophen is a Schedule IV narcotic under the U.S. Controlled Substances Act. Propoxyphene napsylate and acetaminophen can produce drug dependence of the morphine type, and therefore, has the potential for being abused. Psychic dependence, physical dependence and tolerance may develop upon repeated administration. Propoxyphene napsylate and acetaminophen should be prescribed and administered with the same degree of caution appropriate to the use of other narcotic-containing medications.
Abuse
Since propoxyphene napsylate and acetaminophen is a mu-opioid agonist, it may be subject to misuse, abuse, and addiction. Addiction to opioids prescribed for pain management has not been estimated. However, requests for opioids from opioid-addicted patients occur. As such, physicians should take appropriate care in prescribing propoxyphene napsylate and acetaminophen.
Dependence
Opioid analgesics may cause psychological and physical dependence. Physical dependence results in withdrawal symptoms in patients who abruptly discontinue the drug after long term administration. Also, symptoms of withdrawal may be precipitated through the administration of drugs with mu-opioid antagonist activity, e.g., naloxone or mixed agonist/antagonist analgesics (pentazocine, butorphanol, nalbuphine, dezocine) (see OVERDOSAGE ). Physical dependence usually does not occur to a clinically significant degree, until after several weeks of continued opioid usage. Tolerance, in which increasingly larger doses are required to produce the same degree of analgesia, is initially manifested by a shortened duration of an analgesic effect and subsequently, by decreases in the intensity of analgesia.
In chronic pain patients, and in opioid-tolerant cancer patients, the administration of propoxyphene napsylate and acetaminophen should be guided by the degree of tolerance manifested and the doses needed to adequately relieve pain.
The severity of the propoxyphene napsylate and acetaminophen abstinence syndrome may depend on the degree of physical dependence. Withdrawal is characterized by rhinitis, myalgia, abdominal cramping, and occasional diarrhea. Most observable symptoms disappear in 5 to 14 days without treatment; however, there may be a phase of secondary or chronic abstinence which may last for 2 to 6 months characterized by insomnia, irritability, and muscular aches. The patient may be detoxified by gradual reduction of the dose. Gastrointestinal disturbances or dehydration should be treated with supportive care.
Overdosage
Propoxyphene napsylate and acetaminophen is a combination product containing propoxyphene and acetaminophen. Overdose of propoxyphene napsylate and acetaminophen may present with the signs and symptoms of propoxyphene overdose, acetaminophen overdose or both. Fatalities within the first hour of overdosage are not uncommon.
In all cases of suspected overdosage, call your regional Poison Control Center to obtain the most up-to-date information about the treatment of overdose. This recommendation is made because, in general, information regarding the treatment of overdosage may change more rapidly than do package inserts.
Initial consideration should be given to the management of the CNS effects of propoxyphene overdosage. Resuscitative measures should be initiated promptly.
Propoxyphene Overdosage
Symptoms of Propoxyphene Overdosage
The manifestations of acute overdosage with propoxyphene are those of opioid overdosage. The patient is usually somnolent but may be stuporous or comatose and convulsing. Respiratory depression is characteristic. The ventilatory rate and/or tidal volume is decreased, which results in cyanosis and hypoxia. Pupils, initially pinpoint, may become dilated as hypoxia increases. Cheyne-Stokes respiration and apnea may occur. Blood pressure and heart rate are usually normal initially, but blood pressure falls and cardiac performance deteriorates, which ultimately results in pulmonary edema and circulatory collapse, unless the respiratory depression is corrected and adequate ventilation is restored promptly. Cardiac arrhythmias and conduction delay may be present. A combined respiratory-metabolic acidosis occurs owing to retained CO2 (hypercapnia) and to lactic acid formed during anaerobic glycolysis. Acidosis may be severe if large amounts of salicylates have also been ingested. Death may occur.
Treatment of Propoxyphene Overdosage
Attention should be directed first to establishing a patent airway and to restoring ventilation. Mechanically assisted ventilation, with or without oxygen, may be required, and positive pressure respiration may be desirable if pulmonary edema is present. The opioid antagonist naloxone will markedly reduce the degree of respiratory depression, and should be administered promptly, preferably intravenously. The duration of action of the antagonist may be brief. If no response is observed after 10 mg of naloxone have been administered, the diagnosis of propoxyphene toxicity should be questioned.
In addition to the use of an opioid antagonist, the patient may require careful titration with an anticonvulsant to control convulsions. Activated charcoal can adsorb a significant amount of ingested propoxyphene. Dialysis is of little value in poisoning due to propoxyphene. Efforts should be made to determine whether other agents, such as alcohol, barbiturates, tranquilizers, or other CNS depressants, were also ingested, since these increase CNS depression as well as cause specific toxic effects or death.
Acetaminophen Overdosage
Symptoms of Acetaminophen Overdosage
Overdose of acetaminophen may cause dose-dependent potentially fatal hepatic toxicity. Early symptoms within 24 hours after the overdose may include anorexia, nausea, vomiting, diaphoresis, general malaise, and abdominal pain. The patient may then present no symptoms, but evidence of liver dysfunction may become apparent up to 72 hours after ingestion, with elevated serum transaminase and lactic dehydrogenase levels, an increase in serum bilirubin concentrations, and a prolonged prothrombin time. Death from hepatic failure may result 3 to 7 days after overdosage.
Because clinical and laboratory evidence of hepatic toxicity may not be apparent until 48 to 72 hours post-ingestion, liver function studies should be obtained initially and repeated at 24-hour intervals.
Acute renal failure may accompany the hepatic dysfunction and has been noted in patients who do not exhibit signs of fulminant hepatic failure. Typically, renal impairment is more apparent 6 to 9 days after ingestion of the overdose.
Treatment of Acetaminophen Overdosage
In all cases of suspected overdose, immediately call the Rocky Mountain Poison Center's toll-free number (800-525-6115) for assistance in diagnosis and for directions in the use of N-acetylcysteine as an antidote.
Patients' estimates of the quantity of a drug ingested are notoriously unreliable. Therefore, if an acetaminophen overdose is suspected, a serum acetaminophen assay should be obtained as early as possible, but no sooner than 4 hours following ingestion. The antidote, N-acetylcysteine, should be administered as early as possible, and within 16 hours of the overdose ingestion for optimal results.
Dosage And Administration
Propoxyphene napsylate and acetaminophen tablets are intended for the management of mild to moderate pain. The dose should be individually adjusted according to severity of pain, patient response and patient size.
Propoxyphene napsylate and acetaminophen tablets 100 mg/650 mg The usual dosage is one tablet every 4 hours orally as needed for pain. The maximum dose of propoxyphene napsylate and acetaminophen tablets 100 mg/650 mg is 6 tablets per day. Do not exceed the maximum daily dose.
Propoxyphene napsylate and acetaminophen tablets 50 mg/325 mg The usual dosage is two tablets every 4 hours orally as needed for pain. The maximum dose of propoxyphene napsylate and acetaminophen tablets 50 mg/325 mg is 12 tablets per day. Do not exceed the maximum daily dose.
Patients receiving propoxyphene and any CYP3A4 inhibitor should be carefully monitored for an extended period of time and dosage adjustments should be made if warranted.
Consideration should be given to a reduced total daily dosage in elderly patients and in patients with hepatic or renal impairment.
Cessation of Therapy
For patients who used propoxyphene napsylate and acetaminophen on a regular basis for a period of time, when therapy with propoxyphene napsylate and acetaminophen is no longer needed for the treatment of their pain, it may be useful to gradually discontinue the propoxyphene napsylate and acetaminophen over time to prevent the development of an opioid abstinence syndrome (narcotic withdrawal). In general, therapy can be decreased by 25% to 50% per day with careful monitoring for signs and symptoms of withdrawal (see DRUG ABUSE AND DEPENDENCE for description of the signs and symptoms of withdrawal). If the patient develops these signs or symptoms, the dose should be raised to the previous level and titrated down more slowly, either by increasing the interval between decreases, decreasing the amount of change in dose, or both.
How Supplied
Propoxyphene Napsylate and Acetaminophen Tablets, USPÂ 50 mg/325 mg are available as follows:
Orange film coated, unscored, capsule shaped tablets, debossed "5111"Â over "V" on one side and plain on the reverse side.
Propoxyphene Napsylate and Acetaminophen Tablets, USP 100 mg/650 mg are available as follows:
Orange film coated, unscored, capsule shaped tablets, debossed "5112"Â over "V" on one side and plain on the reverse side.
White film coated, unscored, capsule shaped tablets, debossed "5113" over "V" on one side and plain on the reverse side
Pink film coated, unscored, capsule shaped tablets, debossed "5114" over "V" on one side and plain on the reverse side.
They are supplied by Altura Pharmaceuticals, Inc. as follows:
NDC Strength Quantity/Form Color 63874-201-30 100 mg / 650 mg 30 Tablets in a Plastic Bottle Pink, Orange, or White 63874-201-60 100 mg / 650 mg 60 Tablets in a Plastic Bottle Pink, Orange, or White 63874-201-90 100 mg / 650 mg 90 Tablets in a Plastic Bottle Pink, Orange, or White 63874-201-01 100 mg / 650 mg 100 Tablets in a Plastic Bottle Pink, Orange, or White 63874-201-04 100 mg / 650 mg 120 Tablets in a Plastic Bottle Pink, Orange, or White STORAGE AND HANDLING SECTION
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Spl Medguide Section
Inform patients of the availability of a Medication Guide for propoxyphene napsylate and acetaminophen tablets that accompanies each prescription dispensed. Instruct patients to read the propoxyphene napsylate and acetaminophen tablets Medication Guide prior to using propoxyphene napsylate and acetaminophen tablets.
MEDICATION GUIDE
Read this Medication Guide before you start taking propoxyphene napsylate and acetaminophen tablets and each time you get a refill. There may be new information. This information does not take the place of talking to your doctor about your medical condition or your treatment.
What is the most important information I should know about propoxyphene napsylate and acetaminophen tablets?
Propoxyphene napsylate and acetaminophen tablets and other medicines that contain propoxyphene can cause serious side effects, including:
Overdoses by accident or on purpose (intentional overdose). Overdoses with propoxyphene napsylate and acetaminophen tablets may happen when it is taken by itself, or with alcohol or other medicines that can also decrease your breathing and make you very sleepy.
- Death can happen within 1 hour of taking an overdose of propoxyphene napsylate and acetaminophen tablets. Many of the deaths that happen in people who take propoxyphene napsylate and acetaminophen tablets happen in those who:
- have emotional problems
- have thoughts of suicide or attempted suicide, or
- also take antidepressants, sedatives, tranquilizers, muscle relaxants, or other medicines that affect your breathing and make you very sleepy. You should not use any of these medicines with propoxyphene napsylate and acetaminophen tablets without talking to your doctor.
- Before taking propoxyphene napsylate and acetaminophen tablets tell your doctor if you:
- have a lung problem, such as COPD or cor pulmonale
- have liver or kidney problems
- have problems with your pancreas or gallbladder
- have a history of head injury
- are over age 65
- have a history of drug or alcohol abuse or addiction
Take propoxyphene napsylate and acetaminophen tablets exactly as prescribed. Do not change your dose or stop taking propoxyphene napsylate and acetaminophen tablets without first talking to your doctor.
- If you take propoxyphene napsylate and acetaminophen tablets 100 mg/650 mg, do not take more than 6 tablets in one day.
- If you take propoxyphene napsylate and acetaminophen tablets 50 mg/325 mg, do not take more than 12 tablets in one day.
- Before taking propoxyphene napsylate and acetaminophen tablets, tell your doctor about all the medicines you take. Propoxyphene napsylate and acetaminophen tablets and many other medicines may interact with each other and may cause serious side effects. Certain medicines can affect how your liver breaks down other medicines. See "What should I tell my doctor before taking propoxyphene napsylate and acetaminophen tablets?"
- Do not drink grapefruit juice or eat grapefruit while you are taking propoxyphene napsylate and acetaminophen tablets. Grapefruit juice may interact with propoxyphene napsylate and acetaminophen tablets.
- Do not drink alcohol while using propoxyphene napsylate and acetaminophen tablets. Using alcohol with propoxyphene napsylate and acetaminophen tablets may increase your risk of having dangerous side effects.
What is propoxyphene napsylate and acetaminophen tablets?
- Propoxyphene napsylate and acetaminophen tablets is a prescription medicine that contains two medicines: propoxyphene and acetaminophen. Propoxyphene napsylate and acetaminophen tablets is used to relieve mild to moderate pain.
Â
- Propoxyphene napsylate and acetaminophen tablets is a federally controlled substance (C-IV) because it is a strong opioid pain medicine that can be abused by people who abuse prescription medicines or street drugs.
- Prevent theft, misuse or abuse. Keep propoxyphene napsylate and acetaminophen tablets in a safe place to protect it from being stolen. Propoxyphene napsylate and acetaminophen tablets can be a target for people who misuse or abuse prescription medicines or street drugs.
- Never give propoxyphene napsylate and acetaminophen tablets to anyone else, even if they have the same symptoms that you have. It may harm them or even cause death. Selling or giving away this medicine is against the law.
It is not known if propoxyphene napsylate and acetaminophen tablets is safe and effective in children under age 18.
Who should not take propoxyphene napsylate and acetaminophen tablets?
Do not take propoxyphene napsylate and acetaminophen tablets if you:
- are allergic to propoxyphene or acetaminophen. Ask your doctor if you are not sure. See the end of this Medication Guide for a ul of the ingredients in propoxyphene napsylate and acetaminophen tablets.
- are having an asthma attack or have severe asthma, trouble breathing, or lung problems
- have a bowel blockage called paralytic ileus.
What should I tell my doctor before taking propoxyphene napsylate and acetaminophen tablets?
Before taking propoxyphene napsylate and acetaminophen tablets, tell your doctor:
- if you have any of the conditions uled in the section "What is the most important information I should know about propoxyphene napsylate and acetaminophen tablets?"
- if you are allergic to propoxyphene or acetaminophen
- if you plan to have surgery with general anesthesia
- if you are pregnant or plan to become pregnant.
- if you take propoxyphene napsylate and acetaminophen tablets regularly before your baby is born, your newborn baby may have withdrawal symptoms because their body has become used to the medicine. Symptoms of withdrawal in a newborn baby may include:
- irritability
- shaking (tremors)
- jitteriness
- breathing faster than normal
- crying more than usual
- diarrhea or more stools than normal Â
- vomiting
- fever  Â
- if you take propoxyphene napsylate and acetaminophen tablets right before your baby is born, your baby could have breathing problems.
- if you are breast-feeding or plan to breast-feed. Some propoxyphene napsylate and acetaminophen tablets passes into breast milk.
Tell your doctor about all the medicines you take, including prescription, and nonprescription medicines, vitamins, and herbal supplements. Propoxyphene napsylate and acetaminophen tablets interacts with many medicines and may lead to serious side effects. The doses of certain medicines may need to be changed.
Especially tell your doctor if you take: See "What is the most important information I should know about propoxyphene napsylate and acetaminophen tablets?"
- certain medicines that can affect how your liver breaks down other medicines.
- a monoamine oxidase inhibitor (MAOI) medicine
- other medicines that make you sleepy, such as: other medicines for pain, including other opioid medicines, anti-depressant medicines, sleeping pills, anti-anxiety medicines, muscle relaxants, anti-nausea medicines, or tranquilizers
- an anticholinergic medicine
- a water pill (diuretic)
- a medicine for high blood pressure or irregular heart beat
- birth control pills taken by mouth
- lamotrigine (Lamictal, Lamictal CD, Lamictal XR, Lamictal ODT)
- probenecid (Probalan)
- a blood-thinner medicine. You may have an increased risk of bleeding while also taking propoxyphene napsylate and acetaminophen tablets.
- zidovudine (Trizivir, Combivir, Retrovir)
Do not take other medicines that contain acetaminophen while taking propoxyphene napsylate and acetaminophen tablets. See "What are the possible side effects of propoxyphene napsylate and acetaminophen tablets?"
Ask your doctor or pharmacist if you are not sure if your medicine is one uled above.
Know the medicines you take. Keep a ul of them to show to your doctor and pharmacist when you get a new medicine.
How should I take propoxyphene napsylate and acetaminophen tablets? See "What is the most important information I should know about propoxyphene napsylate and acetaminophen tablets?"
- Take propoxyphene napsylate and acetaminophen tablets exactly as prescribed.
- If you take too much propoxyphene napsylate and acetaminophen tablets, or take it with alcohol or other medicines, you may overdose. See "What is the most important information I should know about propoxyphene napsylate and acetaminophen tablets?" You will need medical help right away if you think you have taken an overdose of propoxyphene napsylate and acetaminophen tablets. A large overdose can cause you to become unconscious and die.
Signs and symptoms of an overdose of propoxyphene napsylate and acetaminophen tablets include:
- you are very sleepy or do not respond to others
- confusion
- have trouble breathing or stop breathing
- changes in blood pressure and heart rate
- nausea
- vomiting
- loss of appetite
- stomach-area (abdominal pain)
What are the possible side effects of propoxyphene napsylate and acetaminophen tablets? Propoxyphene napsylate and acetaminophen tablets can cause serious side effects, including: See "What is the most important information I should know about propoxyphene napsylate and acetaminophen tablets?"Â
- Serious breathing problems that can become life-threatening. This is especially true if you already have a serious lung or breathing problem, or your body is not used to opioid pain medicines. This can happen even if you take propoxyphene napsylate and acetaminophen tablets exactly as prescribed by your doctor. Call your doctor or get medical help right away if:
- your breathing slows down
- you have shallow breathing (little chest movement with breathing)
- you feel faint, dizzy, confused, or
- you have any other unusual symptoms
- Propoxyphene napsylate and acetaminophen tablets can cause your blood pressure to drop. This can make you feel dizzy and faint if you get up too fast from sitting or lying down. Low blood pressure is also more likely to happen if you take other medicines that can also lower your blood pressure. Severe low blood pressure can happen if you lose blood or take certain other medicines.
- Liver problems. Propoxyphene napsylate and acetaminophen tablets contains acetaminophen. Acetaminophen can cause serious liver problems if you take more than the recommended dose. Do not take more propoxyphene napsylate and acetaminophen tablets than prescribed. See "How should I take propoxyphene napsylate and acetaminophen tablets?" Do not take any other medicines that contain acetaminophen while also taking propoxyphene napsylate and acetaminophen tablets. Many products contain acetaminophen. Ask your doctor or pharmacist if you are not sure. Liver damage may happen even after symptoms go away. You can die from liver failure days later. Tell your doctor if you have any of these symptoms of a liver problem while taking propoxyphene napsylate and acetaminophen tablets:
- nausea
- vomiting
- loss of appetite
- stomach area (abdominal pain)
- Sleepiness. Propoxyphene napsylate and acetaminophen tablets can cause sleepiness and may affect your ability to make decisions, think clearly, or react quickly. Do not drive, operate heavy machinery, or do other dangerous activities until you know how propoxyphene napsylate and acetaminophen tablets affects you.
- Propoxyphene napsylate and acetaminophen tablets can cause physical dependence if you take it for more than a few weeks. Do not stop taking propoxyphene napsylate and acetaminophen tablets all of a sudden. You could become sick with uncomfortable withdrawal symptoms (for example, nausea, vomiting, diarrhea, anxiety, and shivering) because your body has become used to the medicine. Talk to your doctor about slowly stopping propoxyphene napsylate and acetaminophen tablets to avoid getting sick with withdrawal symptoms. Physical dependence is not the same as drug addiction. Your doctor can tell you more about the differences between physical dependence and drug addiction.
Tell your doctor if you have any of these withdrawal symptoms while you slowly stop taking propoxyphene napsylate and acetaminophen tablets. You may need to stop propoxyphene napsylate and acetaminophen tablets more slowly.
Common side effects of propoxyphene napsylate and acetaminophen tablets include:
- dizziness
- feeling sleepy
- nausea and vomiting
- constipation
- stomach area (abdominal) pain
- skin rashes
- lightheadedness
- headache
- weakness
- feeling of exclient (elation) or discomfort
- seeing, hearing, or sensing things that are not really there (hallucinations)
- blurred vision
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store propoxyphene napsylate and acetaminophen tablets?
- Store propoxyphene napsylate and acetaminophen tablets at 59oF to 86oF (15oC to 30oC).
Keep propoxyphene napsylate and acetaminophen tablets and all medicines out of the reach of children.
General information about propoxyphene napsylate and acetaminophen tablets
Medicines are sometimes prescribed for purposes other than those uled in a Medication Guide. Do not use propoxyphene napsylate and acetaminophen tablets for a purpose for which it was not prescribed. Do not give propoxyphene napsylate and acetaminophen tablets to others even if they have the same symptoms you have. It may harm them and is against the law. This Medication Guide summarizes the most important information about propoxyphene napsylate and acetaminophen tablets. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about propoxyphene napsylate and acetaminophen tablets that is written for health professionals.
What are the ingredients in propoxyphene napsylate and acetaminophen tablets?
Propoxyphene napsylate and acetaminophen tablets 50 mg/325 mg:
Active ingredients: propoxyphene napsylate and acetaminophenInactive ingredients: carnuba wax, D&C Yellow #10 Aluminum Lake, FD&C Red #40 Aluminum Lake, hypromellose, lactose monohydrate, magnesium stearate, polyethylene glycol, polysorbate 80, sodium starch glycolate and titanium dioxide.
Propoxyphene napsylate and acetaminophen tablets 100 mg/650 mg:
Active ingredients: propoxyphene napsylate and acetaminophenInactive ingredients: carnuba wax, hypromellose, lactose monohydrate, magnesium stearate, polyethylene glycol, polysorbate 80, sodium starch glycolate and titanium dioxide. The orange tablets also contain D&C Yellow #10 Aluminum Lake, FD&C Red #40 Aluminum Lake. The pink tablets also contain FD&C Red #40 Aluminum Lake.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Spl Patient Package Insert Section
Manufactured for: QUALITEST PHARMACEUTICALS Huntsville, AL 35811
This Product was Repackaged By:
Altura Pharmaceuticals, Inc. 12540 McCann Drive Santa Fe Springs, CA 90670 United States
Principal Display Panel
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site



