QVAR Dailymed
Generic: beclomethasone dipropionate
Go PRO for all pill images
Description
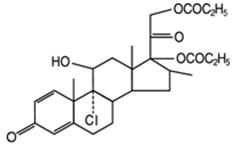
The active component of QVAR 40 mcg Inhalation Aerosol and QVAR 80 mcg Inhalation Aerosol is beclomethasone dipropionate, USP, an anti-inflammatory corticosteroid having the chemical name 9-chloro-11β,17,21-trihydroxy-16β-methylpregna-1,4-diene-3,20-dione 17,21-dipropionate. Beclomethasone dipropionate (BDP) is a diester of beclomethasone, a synthetic corticosteroid chemically related to dexamethasone. Beclomethasone differs from dexamethasone in having a chlorine at the 9-alpha carbon in place of a fluorine, and in having a 16 beta-methyl group instead of a 16 alpha-methyl group. Beclomethasone dipropionate is a white to creamy white, odorless powder with a molecular formula of C28H37ClO7 and a molecular weight of 521.1. Its chemical structure is:
QVAR is a pressurized, metered-dose aerosol intended for oral inhalation only. Each unit contains a solution of beclomethasone dipropionate in propellant HFA-134a (1,1,1,2 tetrafluoroethane) and ethanol. QVAR 40 mcg delivers 40 mcg of beclomethasone dipropionate from the actuator and 50 mcg from the valve. QVAR 80 mcg delivers 80 mcg of beclomethasone dipropionate from the actuator and 100 mcg from the valve. Both products deliver 50 microliters (59 milligrams) of solution formulation from the valve with each actuation. Each canister provides 100 inhalations. QVAR should be "primed" or actuated twice prior to taking the first dose from a new canister, or when the inhaler has not been used for more than ten days. Avoid spraying in the eyes or face while priming QVAR. This product does not contain chlorofluorocarbons (CFCs).
Clinical Pharmacology
Airway inflammation is known to be an important component in the pathogenesis of asthma. Inflammation occurs in both large and small airways. Corticosteroids have multiple anti-inflammatory effects, inhibiting both inflammatory cells (e.g., mast cells, eosinophils, basophils, lymphocytes, macrophages, and neutrophils) and release of inflammatory mediators (e.g., histamine, eicosanoids, leukotrienes, and cytokines). These anti-inflammatory actions of corticosteroids such as beclomethasone dipropionate contribute to their efficacy in asthma.
Beclomethasone dipropionate is a prodrug that is rapidly activated by hydrolysis to the active monoester, 17 monopropionate (17-BMP). Beclomethasone 17 monopropionate has been shown in vitro to exhibit a binding affinity for the human glucocorticoid receptor which is approximately 13 times that of dexamethasone, 6 times that of triamcinolone acetonide, 1.5 times that of budesonide and 25 times that of beclomethasone dipropionate. The clinical significance of these findings is unknown.
Studies in patients with asthma have shown a favorable ratio between topical anti-inflammatory activity and systemic corticosteroid effects with recommended doses of QVAR.
Pharmacokinetics
Beclomethasone dipropionate (BDP) undergoes rapid and extensive conversion to beclomethasone-17-monopropionate (17-BMP) during absorption. The pharmacokinetics of 17-BMP has been studied in asthmatics given single doses.
Absorption
The mean peak plasma concentration (Cmax) of BDP was 88 pg/ml at 0.5 hour after inhalation of 320 mcg using QVAR (four actuations of the 80 mcg/actuation strength). The mean peak plasma concentration of the major and most active metabolite, 17-BMP, was 1419 pg/ml at 0.7 hour after inhalation of 320 mcg of QVAR. When the same nominal dose is provided by the two QVAR strengths (40 and 80 mcg/actuation), equivalent systemic pharmacokinetics can be expected. The Cmax of 17-BMP increased dose proportionally in the dose range of 80 and 320 mcg.
Metabolism
Three major metabolites are formed via cytochrome P450 3A catalyzed biotransformation - beclomethasone-17-monopropionate (17-BMP), beclomethasone-21-monopropionate (21-BMP) and beclomethasone (BOH). Lung slices metabolize BDP rapidly to 17-BMP and more slowly to BOH. 17-BMP is the most active metabolite.
Distribution
The in vitro protein binding for 17-BMP was reported to be 94-96% over the concentration range of 1000 to 5000 pg/mL. Protein binding was constant over the concentration range evaluated. There is no evidence of tissue storage of BDP or its metabolites.
Elimination
The major route of elimination of inhaled BDP appears to be via hydrolysis. More than 90% of inhaled BDP is found as 17-BMP in the systemic circulation. The mean elimination half-life of 17-BMP is 2.8 hours. Irrespective of the route of administration (injection, oral or inhalation), BDP and its metabolites are mainly excreted in the feces. Less than 10% of the drug and its metabolites are excreted in the urine.
Special Populations
Formal pharmacokinetic studies using QVAR were not conducted in any special populations.
Pediatrics
The pharmacokinetics of 17-BMP, including dose and strength proportionalities, is similar in children and adults, although the exposure is highly variable. In 17 children (mean age 10 years), the Cmax of 17-BMP was 787 pg/ml at 0.6 hour after inhalation of 160 mcg (four actuations of the 40 mcg/actuation strength of HFA beclomethasone dipropionate). The systemic exposure to 17-BMP from 160 mcg of HFA-BDP administered without a spacer was comparable to the systemic exposure to 17-BMP from 336 mcg CFC-BDP administered with a large volume spacer in 14 children (mean age 12 years). This implies that approximately twice the systemic exposure to 17-BMP would be expected for comparable mg doses of HFA-BDP without a spacer and CFC-BDP with a large volume spacer.
Pharmacodynamics
Improvement in asthma control following inhalation can occur within 24 hours of beginning treatment in some patients, although maximum benefit may not be achieved for 1 to 2 weeks, or longer. The effects of QVAR on the hypothalamic-pituitary-adrenal (HPA) axis were studied in 40 corticosteroid naive patients. QVAR, at doses of 80, 160 or 320 mcg twice daily was compared with placebo and 336 mcg twice daily of beclomethasone dipropionate in a CFC propellant based formulation (CFC-BDP). Active treatment groups showed an expected dose-related reduction in 24-hour urinary free cortisol (a sensitive marker of adrenal production of cortisol). Patients treated with the highest recommended dose of QVAR (320 mcg twice daily) had a 37.3% reduction in 24-hour urinary free cortisol compared to a reduction of 47.3% produced by treatment with 336 mcg twice daily of CFC-BDP. There was a 12.2% reduction in 24 hour urinary free cortisol seen in the group of patients that received 80 mcg twice daily of QVAR and a 24.6% reduction in the group of patients that received 160 mcg twice daily. An open label study of 354 asthma patients given QVAR at recommended doses for one year assessed the effect of QVAR treatment on the HPA axis (as measured by both morning and stimulated plasma cortisol). Less than 1% of patients treated for one year with QVAR had an abnormal response (peak less than 18 mcg/dL) to short-cosyntropin test.
Clinical Trials
Blinded, randomized, parallel, placebo-controlled and active-controlled clinical studies were conducted in 940 adult asthma patients to assess the efficacy and safety of QVAR in the treatment of asthma. Fixed doses ranging from 40 mcg to 160 mcg twice daily were compared to placebo, and doses ranging from 40 mcg to 320 mcg twice daily were compared with doses of 42 mcg to 336 mcg twice daily of an active CFC-BDP comparator. These studies provided information about appropriate dosing through a range of asthma severity. A blinded, randomized, parallel, placebo-controlled study was conducted in 353 pediatric patients (age 5-12 years) to assess the efficacy and safety of HFA beclomethasone dipropionate in the treatment of asthma. Fixed doses of 40 mcg and 80 mcg twice daily were compared with placebo in this study. In these adult and pediatric efficacy trials, at the doses studied, measures of pulmonary function [forced expiratory volume in 1 second (FEV1) and morning peak expiratory flow (AM PEF)] and asthma symptoms were significantly improved with QVAR treatment when compared to placebo.
In controlled clinical trials with adult patients not adequately controlled with beta-agonist alone, QVAR was effective at improving asthma control at doses as low as 40 mcg twice daily (80 mcg/day). Comparable asthma control was achieved at lower daily doses of QVAR than with CFC-BDP. Treatment with increasing doses of both QVAR and CFC-BDP generally resulted in increased improvement in FEV1. In this trial the improvement in FEV1 across doses was greater for QVAR than for CFC-BDP, indicating a shift in the dose response curve for QVAR.
Patients Not Previously Receiving Corticosteroid Therapy
In a 6 week clinical trial, 270 steroid naive patients with symptomatic asthma being treated with as-needed beta-agonist bronchodilators, were randomized to receive either 40 mcg twice daily of QVAR, 80 mcg twice daily of QVAR, or placebo. Both doses of QVAR were effective in improving asthma control with significantly greater improvements in FEV1, AM PEF, and asthma symptoms than with placebo. Shown below is the change from baseline in AM PEF during this trial.
A 6-Week Clinical Trial in Patients with Mild to Moderate Asthma Not onCorticosteroid Therapy Prior to Study Entry:Mean Change in AM PEF 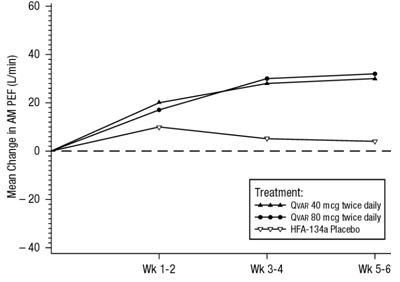
In a 6-week clinical trial, 256 patients with symptomatic asthma being treated with as-needed beta-agonist bronchodilators, were randomized to receive either 160 mcg twice daily of QVAR (delivered as either 40 mcg/actuation or 80 mcg/actuation) or placebo. Treatment with QVAR significantly improved asthma control, as assessed by FEV1, AM PEF, and asthma symptoms, when compared to treatment with placebo. Comparable improvement in AM PEF was seen for patients receiving 160 mcg twice daily QVAR from the 40 mcg and 80 mcg strength products.
Patients Responsive to a Short Course of Oral Corticosteroids
In another clinical trial, 347 patients with symptomatic asthma, being treated with as-needed inhaled beta-agonist bronchodilators and, in some cases, inhaled corticosteroids, were given a 7-12 day course of oral corticosteroids and then randomized to receive either 320 mcg daily of QVAR, 672 mcg of CFC-BDP, or placebo. Patients treated with either QVAR or CFC-BDP had significantly better asthma control, as assessed by AM PEF, FEV1 and asthma symptoms, and fewer study withdrawals due to asthma symptoms, than those treated with placebo over 12 weeks of treatment. A daily dose of 320 mcg QVAR administered in divided doses provided comparable control of AM PEF and FEV1 as 672 mcg of CFC-BDP. Shown below are the mean AM PEF results from this trial.
A 12-Week Clinical Trial in Moderate Symptomatic Patients withAsthma Responding to Oral Corticosteroid Therapy: Mean AM PEF by Study Week 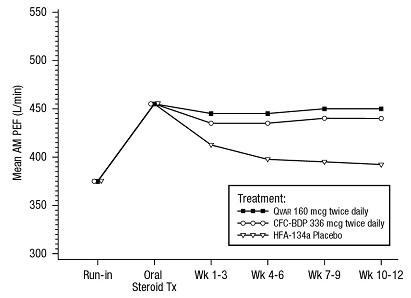
Patients Previously on Inhaled Corticosteroids
In a 6-week clinical trial, 323 patients, who exhibited a deterioration in asthma control during an inhaled corticosteroid washout period, were randomized to daily treatment with either 40, 160, or 320 mcg twice daily QVAR or 42, 168, or 336 mcg twice daily CFC-BDP. Treatment with increasing doses of both QVAR and CFC-BDP resulted in increased improvement in FEV1, FEF25-75% (forced expiratory flow over 25-75% of the vital capacity), and asthma symptoms. Shown below is the change from baseline in FEV1 as percent predicted after 6 weeks of treatment.
A 6-Week Dose Response Clinical Trial in Patients with Inhaled Corticosteroid Dependent Asthma:Mean Change in FEV1 as Percent of Predicted 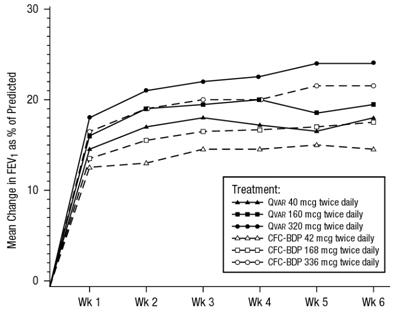
Patients Previously Maintained on Oral Corticosteroids
Clinical experience has shown that some patients with asthma who require oral corticosteroid therapy for control of symptoms can be partially or completely withdrawn from oral corticosteroids if therapy with beclomethasone dipropionate aerosol is substituted. Inhaled corticosteroids may not be effective for all patients with asthma or at all stages of the disease in a given patient.
Pediatric Experience
In one 12-week clinical trial, pediatric patients (age 5-12 years) with symptomatic asthma (N=353) being treated with as-needed beta-agonist bronchodilators were randomized to receive either 40 mcg or 80 mcg twice daily of HFA beclomethasone dipropionate or placebo. Both doses were effective in improving asthma control with significantly greater improvements in FEV1 (9% and 10% predicted change from baseline at week 12 in FEV1 percent predicted, respectively) than with placebo (4% predicted change).
Indications And Usage
QVAR is indicated in the maintenance treatment of asthma as prophylactic therapy in patients 5 years of age and older. QVAR is also indicated for asthma patients who require systemic corticosteroid administration, where adding QVAR may reduce or eliminate the need for the systemic corticosteroids.
Beclomethasone dipropionate is NOT indicated for the relief of acute bronchospasm.
Contraindications
QVAR is contraindicated in the primary treatment of status asthmaticus or other acute episodes of asthma where intensive measures are required. Hypersensitivity to any of the ingredients of this preparation contraindicates its use.
Warnings
Particular care is needed in patients who are transferred from systemically active corticosteroids to QVAR because deaths due to adrenal insufficiency have occurred in asthmatic patients during and after transfer from systemic corticosteroids to less systemically available inhaled corticosteroids. After withdrawal from systemic corticosteroids, a number of months are required for recovery of hypothalamic-pituitary-adrenal (HPA) function.
Patients who have been previously maintained on 20 mg or more per day of prednisone (or its equivalent) may be most susceptible, particularly when their systemic corticosteroids have been almost completely withdrawn. During this period of HPA suppression, patients may exhibit signs and symptoms of adrenal insufficiency when exposed to trauma, surgery, or infections (particularly gastroenteritis) or other conditions with severe electrolyte loss. Although QVAR may provide control of asthmatic symptoms during these episodes, in recommended doses it supplies less than normal physiological amounts of glucocorticoid systemically and does NOT provide the mineralocorticoid that is necessary for coping with these emergencies.
During periods of stress or a severe asthmatic attack, patients who have been withdrawn from systemic corticosteroids should be instructed to resume oral corticosteroids (in large doses) immediately and to contact their physician for further instruction. These patients should also be instructed to carry a warning card indicating that they may need supplementary systemic steroids during periods of stress or a severe asthma attack.
Transfer of patients from systemic steroid therapy to QVAR may unmask allergic conditions previously suppressed by the systemic steroid therapy, e.g., rhinitis, conjunctivitis, and eczema.
Persons who are on drugs which suppress the immune system are more susceptible to infections than healthy individuals. Chickenpox and measles, for example, can have a more serious or even fatal course in non-immune children or adults on corticosteroids. In such children or adults who have not had these diseases or been properly immunized, particular care should be taken to avoid exposure. It is not known how the dose, route and duration of corticosteroid administration affects the risk of developing a disseminated infection. Nor is the contribution of the underlying disease and/or prior corticosteroid treatment known. If exposed to chickenpox, prophylaxis with varicella-zoster immune globulin (VZIG) may be indicated. If exposed to measles, prophylaxis with pooled intramuscular immunoglobulin (IG) may be indicated. (See the respective package inserts for complete VZIG and IG prescribing information.) If chickenpox develops, treatment with antiviral agents may be considered.
QVAR is not a bronchodilator and is not indicated for rapid relief of bronchospasm.
As with other inhaled asthma medications, bronchospasm, with an immediate increase in wheezing, may occur after dosing. If bronchospasm occurs following dosing with QVAR, it should be treated immediately with a short acting inhaled bronchodilator. Treatment with QVAR should be discontinued and alternate therapy instituted. Patients should be instructed to contact their physician immediately when episodes of asthma, which are not responsive to bronchodilators, occur during the course of treatment with QVAR. During such episodes, patients may require therapy with oral corticosteroids.
Precautions
General
During withdrawal from oral corticosteroids, some patients may experience symptoms of systemically active corticosteroid withdrawal, e.g., joint and/or muscular pain, lassitude and depression, despite maintenance or even improvement of respiratory function. Although suppression of HPA function below the clinical normal range did not occur with doses of QVAR up to and including 640 mcg/day, a dose dependent reduction of adrenal cortisol production was observed. Since inhaled beclomethasone dipropionate is absorbed into the circulation and can be systemically active, HPA axis suppression by QVAR could occur when recommended doses are exceeded or in particularly sensitive individuals. Since individual sensitivity to effects on cortisol production exist, physicians should consider this information when prescribing QVAR. Because of the possibility of systemic absorption of inhaled corticosteroids, patients treated with these drugs should be observed carefully for any evidence of systemic corticosteroid effect. Particular care should be taken in observing patients postoperatively or during periods of stress for evidence of inadequate adrenal response.
It is possible that systemic corticosteroid effects, such as hypercorticism and adrenal suppression, may appear in a small number of patients, particularly at higher doses. If such changes occur, QVAR should be reduced slowly, consistent with accepted procedures for management of asthma symptoms and for tapering of systemic steroids.
A 12 month randomized controlled clinical trial evaluated the effects of HFA beclomethasone dipropionate without spacer versus CFC beclomethasone dipropionate with large volume spacer on growth in children age 5-11. A total of 520 patients were enrolled, of whom 394 received HFA-BDP (100 – 400 mcg/day ex-valve) and 126 received CFC-BDP (200 – 800 mcg/day ex-valve). Similar control of asthma was noted in each treatment arm. When comparing results at month 12 to baseline, the mean growth velocity in children treated with HFA-BDP was approximately 0.5 cm/year less than that noted with children treated with CFC-BDP via large volume spacer.
A reduction in growth velocity in growing children may occur as a result of inadequate control of chronic diseases such as asthma or from use of corticosteroids for treatment. Physicians should closely follow the growth of all pediatric patients taking corticosteroids by any route and weigh the benefits of corticosteroid therapy and asthma control against the possibility of growth suppression.
The long-term and systemic effects of QVAR in humans are still not fully known. In particular, the effects resulting from chronic use of the agent on developmental or immunologic processes in the mouth, pharynx, trachea, and lung are unknown.
Inhaled corticosteroids should be used with caution, if at all, in patients with active or quiescent tuberculosis infection of the respiratory tract; untreated systemic fungal, bacterial, parasitic or viral infections; or ocular herpes simplex.
Rare instances of glaucoma, increased intraocular pressure, and cataracts have been reported following the inhaled administration of corticosteroids.
Information for Patients
Patients being treated with QVAR should receive the following information and instructions. This information is intended to aid them in the safe and effective use of this medication. It is not a disclosure of all possible adverse or intended effects.
Persons who are on immunosuppressant doses of corticosteroids should be warned to avoid exposure to chickenpox or measles. Patients should also be advised that if they are exposed to these diseases, medical advice should be sought without delay.
Patients should use QVAR at regular intervals as directed. Results of clinical trials indicated significant improvements may occur within the first 24 hours of treatment in some patients; however, the full benefit may not be achieved until treatment has been administered for 1 to 2 weeks, or longer. The patient should not increase the prescribed dosage but should contact their physician if symptoms do not improve or if the condition worsens.
Patients should be advised that QVAR is not intended for use in the treatment of acute asthma. The patient should be instructed to contact their physician immediately if there is any deterioration of their asthma.
Patients should be instructed on the proper use of their inhaler. Patients may wish to rinse their mouth after QVAR use. The patient should also be advised that QVAR may have a different taste and inhalation sensation than that of an inhaler containing CFC propellant.
QVAR use should not be stopped abruptly. The patient should contact their physician immediately if use of QVAR is discontinued.
For the proper use of QVAR, the patient should read and carefully follow the accompanying Patient's Instructions.
Carcinogenesis, Mutagenesis, Impairment of Fertility
The carcinogenicity of beclomethasone dipropionate was evaluated in rats which were exposed for a total of 95 weeks, 13 weeks at inhalation doses up to 0.4 mg/kg/day and the remaining 82 weeks at combined oral and inhalation doses up to 2.4 mg/kg/day. There was no evidence of carcinogenicity in this study at the highest dose, which is approximately 30 and 55 times the maximum recommended daily inhalation dose in adults and children, respectively, on a mg/m2 basis.
Beclomethasone dipropionate did not induce gene mutation in the bacterial cells or mammalian Chinese Hamster ovary (CHO) cells in vitro. No significant clastogenic effect was seen in cultured CHO cells in vitro or in the mouse micronucleus test in vivo.
In rats, beclomethasone dipropionate caused decreased conception rates at an oral dose of 16 mg/kg/day (approximately 200 times the maximum recommended daily inhalation dose in adults on a mg/m2 basis). Impairment of fertility, as evidence by inhibition of the estrous cycle in dogs, was observed following treatment by the oral route at a dose of 0.5 mg/kg/day (approximately 20 times the maximum recommended daily inhalation dose in adults on a mg/m2 basis). No inhibition of the estrous cycle in dogs was seen following 12 months of exposure to beclomethasone dipropionate by the Inhalation route at an estimated daily dose of 0.33 mg/kg (approximately 15 times the maximum recommended daily inhalation dose in adults on a mg/m2 basis).
Pregnancy Teratogenic Effects Pregnancy Category C
Like other corticosteroids, parenteral (subcutaneous) beclomethasone dipropionate was teratogenic and embryocidal in the mouse and rabbit when given at a dose of 0.1 mg/kg/day in mice or at a dose of 0.025 mg/kg/day in rabbits. These doses in mice and rabbits were approximately one-half the maximum recommended daily inhalation dose in adults on a mg/m2 basis. No teratogenicity or embryocidal effects were seen in rats when exposed to an inhalation dose of 15 mg/kg/day (approximately 190 times the maximum recommended daily inhalation dose in adults on a mg/m2 basis). There are no adequate and well controlled studies in pregnant women. Beclomethasone dipropionate should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Non-teratogenic Effects
Findings of drug-related adrenal toxicity in fetuses following administration of beclomethasone dipropionate to rats suggest that infants born of mothers receiving substantial doses of QVAR during pregnancy should be observed for adrenal suppression.
Nursing Mothers
Corticosteroids are secreted in human milk. Because of the potential for serious adverse reactions in nursing infants from QVAR, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Eight-hundred and thirty-four children between the ages of 5 and 12 were treated with HFA beclomethasone dipropionate (HFA BDP) in clinical trials. The safety and effectiveness of QVAR in children below 5 years of age have not been established.
Use of QVAR with a spacer device in children less than 5 years of age is not recommended. In vitro dose characterization studies were performed with QVAR 40 mcg/actuation with the Optichamber and AeroChamber Plus® spacer utilizing inspiratory flows representative of children under 5 years old. These studies indicated that the amount of medication delivered through the spacing device decreased rapidly with increasing wait times of 5 to 10 seconds as shown in Table 1. If QVAR is used with a spacer device, it is important to inhale immediately.
Based on the average inspiratory flow rates generated by children 6 months to 5 years old, the projected daily dose derived from QVAR 40 mcg at one puff per day at various wait times is depicted in the table below:
TABLE 1 Wait time, seconds Mean medication delivery through AeroChamber, mcg/actuation (*) Body Weight 50th percentile, kg (**) Medication delivered per dose, mcg/kg (***) Age 6 months, Flow rate 4.8 L/min 0 11.5 7.6 1.2 Age 2 years, Flow rate 8.2 L/min 0 14.1 13.5 0.83 Age 2 years, Flow rate 8.2 L/min 5 5.4 13.5 0.32 Age 2 years, Flow rate 8.2 L/min 10 3.9 13.5 0.23 Age 5 years, Flow rate 11.0 L/min 0 17.5 18 0.78
(*) Summary Report; Pediatric Dose Characterization of QVAR with Spacer; 3M Pharmaceutical Development, July 21, 2004.
(**) CDC Growth charts, developed by the National Center for Health Statistics in collaboration with the National Center for Chronic Disease Prevention and Health Promotion (2000).
(***) Includes an estimated 20% loss in the masks
(***) QVAR 40mcg in an average adult without using a spacer delivers approximately 0.4 mcg/kg, or bid, 0.8 mcg/kg/day.
Oral inhaled corticosteroids have been shown to cause a reduction in growth velocity in children and teenagers with extended use. If a child or teenager on any corticosteroid appears to have growth suppression, the possibility that they are particularly sensitive to this effect of corticosteroids should be considered (see PRECAUTIONS, General).
Geriatric Use
Clinical studies of QVAR did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Adverse Reactions
The following reporting rates of common adverse experiences are based upon four clinical trials in which 1196 Patients (671 female and 525 male adults previously treated with as-needed bronchodilators and/or inhaled corticosteroids) were treated with QVAR (doses of 40, 80, 160, or 320 mcg twice daily) or CFC-BDP (doses of 42, 168, or 336 mcg twice daily) or placebo. The table below includes all events reported by patients taking QVAR (whether considered drug related or not) that occurred at a rate over 3% for either QVAR or CFC-BDP. In considering these data, difference in average duration of exposure and clinical trial design should be taken into account.
Adverse Events Reported by at Least 3% of the Patients for Either QVAR or CFC-BDP by Treatment and Daily Dose QVAR CFC-BDP Adverse Events Placebo(N=289)% Total(N=624)% 80-160mcg(N=233)% 320mcg(N=335)% 640mcg(N=56)% Total(N=283)% 84mcg(N=59)% 336mcg(N=55)% 672mcg(N=169)% HEADACHE 9 12 15 8 25 15 14 11 17 PHARYNGITIS 4 8 6 5 27 10 12 9 10 UPPER RESP TRACT INFECTION 11 9 7 11 5 12 3 9 17 RHINITIS 9 6 8 3 7 11 15 9 10 INCREASED ASTHMA SYMPTOMS 18 3 2 4 0 8 14 5 7 ORAL SYMPTOMS 2 3 3 3 2 6 7 5 5 INHALATION ROUTE SINUSITIS 2 3 3 3 0 4 7 2 4 PAIN less than 1 2 1 2 5 3 3 5 2 BACK PAIN 1 1 2 less than 1 4 4 2 4 4 NAUSEA 0 1 less than 1 1 2 3 5 5 1 DYSPHONIA 2 less than 1 1 0 4 4 0 0 6
Other adverse events that occurred in these clinical trials using QVAR with an incidence of 1% to 3% and which occurred at a greater incidence than placebo were: dysphonia, dysmenorrhea and coughing.
No patients treated with QVAR in the clinical development program developed symptomatic oropharyngeal candidiasis.
If such an infection develops, treatment with appropriate antifungal therapy or discontinuance of treatment with QVAR may be required.
Pediatric Studies
In two 12-week placebo controlled studies in steroid naive pediatric patients 5 to 12 years of age, no clinically relevant differences were found in the pattern, severity, or frequency of adverse events compared with those reported in adults, with the exception of conditions which are more prevalent in a pediatric population generally.
Adverse Event Reports from Other Sources
Rare cases of immediate and delayed hypersensitivity reactions, including urticaria, angioedema, rash, and bronchospasm, have been reported following the oral and intranasal inhalation of beclomethasone dipropionate.
Overdosage
There were no deaths over 15 days following the oral administration of a single dose of 3000 mg/kg in mice, 2000 mg/kg in rats, and 1000 mg/kg in rabbits. The doses in mice, rats, and rabbits were 19,000, 25,000, and 25,000 times, respectively, the maximum recommended daily inhalation in adults or 36,000, 48,000, and 48,000 times, respectively the maximum recommended daily inhalation dose in children on a mg/m2 basis.
Dosage And Administration
Patients should prime QVAR by actuating into the air twice before using for the first time or if QVAR has not been used for over ten days. Avoid spraying in the eyes or face when priming QVAR. QVAR is a solution aerosol, which does not require shaking. Consistent dose delivery is achieved, whether using the 40 or 80 mcg strengths, due to proportionality of the two products (i.e., two actuations of 40 mcg strength should provide a dose comparable to one actuation of the 80 mcg strength).
QVAR should be administered by the oral inhaled route in patients 5 years of age and older. Use of QVAR with a spacer device in children less than 5 years of age is not recommended (see PRECAUTIONS, Pediatric Use). The onset and degree of symptom relief will vary in individual patients. Improvement in asthma symptoms should be expected within the first or second week of starting treatment, but maximum benefit should not be expected until 3-4 weeks of therapy. For patients who do not respond adequately to the starting dose after 3-4 weeks of therapy, higher doses may provide additional asthma control. The safety and efficacy of QVAR when administered in excess of recommended doses has not been established.
Recommended Dosage for QVAR:
Previous Therapy Recommended Starting Dose Highest Recommended Dose Adults and Adolescents: Bronchodilators Alone 40 to 80 mcg twice daily 320 mcg twice daily Inhaled Corticosteroids 40 to 160 mcg twice daily 320 mcg twice daily Children 5 to 11 years: Bronchodilators Alone 40 mcg twice daily 80 mcg twice daily Inhaled Corticosteroids 40 mcg twice daily 80 mcg twice daily
As with any inhaled corticosteroid, physicians are advised to titrate the dose of QVAR downward over time to the lowest level that maintains proper asthma control. This is particularly important in children since a controlled study has shown that QVAR has the potential to affect growth in children. Patients should be instructed on the proper use of their inhaler.
Patients Not Receiving Systemic Corticosteroids
Patients who require maintenance therapy of their asthma may benefit from treatment with QVAR at the doses recommended above. In patients who respond to QVAR, improvement in pulmonary function is usually apparent within 1 to 4 weeks after the start of therapy. Once the desired effect is achieved, consideration should be given to tapering to the lowest effective dose.
Patients Maintained on Systemic Corticosteroids
QVAR may be effective in the management of asthmatics maintained on systemic corticosteroids and may permit replacement or significant reduction in the dosage of systemic corticosteroids.
The patient's asthma should be reasonably stable before treatment with QVAR is started. Initially, QVAR should be used concurrently with the patient's usual maintenance dose of systemic corticosteroids. After approximately one week, gradual withdrawal of the systemic corticosteroids is started by reducing the daily or alternate daily dose. Reductions may be made after an interval of one or two weeks, depending on the response of the patient. A slow rate of withdrawal is strongly recommended. Generally these decrements should not exceed 2.5 mg of prednisone or its equivalent. During withdrawal, some patients may experience symptoms of systemic corticosteroid withdrawal, e.g. joint and/or muscular pain, lassitude and depression, despite maintenance or even improvement in pulmonary function. Such patients should be encouraged to continue with the inhaler but should be monitored for objective signs of adrenal insufficiency. If evidence of adrenal insufficiency occurs, the systemic corticosteroid doses should be increased temporarily and thereafter withdrawal should continue more slowly.
During periods of stress or a severe asthma attack, transfer patients may require supplementary treatment with systemic corticosteroids.
DIRECTIONS FOR USE
Illustrated Patient's Instructions for proper use accompany each package of QVAR.
How Supplied
QVAR is supplied in two strengths:
QVAR 40 mcg is supplied in a 7.3 g canister containing 100 actuations with a beige plastic actuator and gray dust cap, and Patient's Instructions; box of one; 100 Actuations – NDC 54868-5857-0 or in an 8.7 g canister containing 120 actuations with a beige plastic actuator and gray dust cap, and Patient's Instructions; box of one;120 Actuations - NDC 54868-5857-1 QVAR 80 mcg is supplied in a 7.3 g canister containing 100 actuations with a dark mauve plastic actuator and gray dust cap, and Patient's Instructions; box of one; 100 Actuations – NDC 54868-5858-0 or in an 8.7 g canister containing 120 actuations with a dark mauve plastic actuator and gray dust cap, and Patient's Instructions; box of one;120 Actuations - NDC 54868-5858-1
The correct amount of medication in each inhalation cannot be assured after 100 actuations from the 7.3 g canister or 120 actuations from the 8.7 g canister even though the canister is not completely empty. The canister should be discarded when the labeled number of actuations have been used.
Store QVAR Inhalation Aerosol when not being used, so that the product rests on the concave end of the canister with the plastic actuator on top.
Store at 25°C (77°F).
Excursions between 15° and 30°C (59° and 86°F) are permitted (see USP). For optimal results, the canister should be at room temperature when used. QVAR Inhalation Aerosol canister should only be used with the QVAR Inhalation Aerosol actuator and the actuator should not be used with any other inhalation drug product.
CONTENTS UNDER PRESSURE
Do not puncture. Do not use or store near heat or open flame. Exposure to temperatures above 49°C (120°F) may cause bursting. Never throw container into fire or incinerator.
Keep out of reach of children.
Rx only
U.S. Patent Nos. 5605674, 5683677, 5695743, 5776432
Mktd by:Teva Respiratory, LLC– Horsham, PA 19044
September, 2010
Developed and Manufactured by:3M Drug Delivery SystemNorthridge, CA 91324 OR 3M Health Care, Ltd.Loughborough, UK
© 2010 Teva Respiratory, LLC639300QVAR® is a registered trademark of IVAX LLC, a member of the TEVA Group.Rev. 09/10
OptiChamber is a registered trademark of Respironics Healthscan, Inc. and AeroChamber Plus is a registered trademark of Trudell Medical International Trudell Partnership Holdings Limited and Packard Medical Supply Centre Ltd.
Relabeling of "Additional" barcode label by: Physicians Total Care, Inc.Tulsa, OK 74146
Patient's Instructions
QVAR® (beclomethasone dipropionate HFA)INHALATION AEROSOL
Attention Pharmacist: Detach "Patient's Instructions for Use" from package insert and dispense with the product.
PATIENT'S INSTRUCTIONS
It is important that you read these instructions before using QVAR.
Correct and regular use of the inhaler will prevent or lessen the severity of asthma attacks.
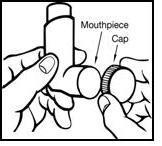
1. Remove the plastic cap (see Figure 1) and be sure there are no foreign objects in the mouthpiece.
Figure 1
2. As with all aerosol medications, it is recommended to prime the QVAR inhaler before using for the very first time after purchase, and in cases where the inhaler has not been used for more than ten days. Prime by releasing two actuations into the air, away from your eyes and face. Be sure the canister is firmly seated in the plastic mouthpiece adapter before each use.
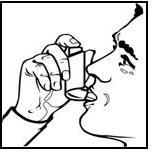
3. BREATHE OUT AS FULLY AS YOU COMFORTABLY CAN. Hold the inhaler as shown in Figure 2. Close your lips around the mouthpiece, keeping your tongue below it.
Figure 2
4. WHILE BREATHING IN DEEPLY AND SLOWLY, PRESS DOWN ON THE CAN WITH YOUR FINGER. When you have finished breathing in, hold your breath as long as you comfortably can (i.e., 5 - 10 seconds).
5. TAKE YOUR FINGER OFF THE CAN and remove the inhaler from your mouth. Breathe out gently.
6. If your physician has told you to take more than one inhalation per treatment repeat steps 3 through 5.
7. You should rinse your mouth with water after treatment.
8. For normal hygiene, the mouthpiece of your inhaler should be cleaned weekly with a clean, dry tissue or cloth.
DO NOT WASH OR PUT ANY PART OF YOUR INHALER IN WATER.
9. DISCARD THE CANISTER AFTER the date calculated by your physician or pharmacist. The correct amount of medication in each inhalation cannot be assured after 100 actuations from the 7.3 g canister even though the canister is not completely empty. The canister should be discarded when the labeled number of actuations have been used. Before the discard date you should consult your physician to determine whether a refill is needed. It is advisable to keep track of the number of doses taken from the canister to better predict when a refill is necessary. Just as you should not take extra doses without consulting your physician, you also should not stop taking QVAR without consulting your physician.
IMPORTANT QVAR is preventive therapy for asthma and must be used regularly and at the times your physician has prescribed.
DO NOT CONFUSE QVAR WITH OTHER ASTHMA MEDICATION. QVAR WILL NOT PROVIDE IMMEDIATE RELIEF IF YOU ARE HAVING AN ASTHMA ATTACK.
Your physician will decide whether other medication is needed should you require immediate relief. If you also use another medicine by inhalation, you should consult your physician for instructions on when to use it in relation to using QVAR. If this is the first time you will be using QVAR, it may take from 1 to 4 weeks before you feel the full benefits.
QVAR Inhalation Aerosol canister should only be used with the QVAR Inhalation Aerosol mouthpiece and the mouthpiece should not be used with any other inhalation drug product.
DOSAGE
Use only as directed by your physician.
CONTENTS UNDER PRESSURE
Do not puncture. Do not use or store near heat or open flame. Exposure to temperatures above 49°C (120°F) may cause bursting. Never throw container into fire or incinerator.
Keep out of reach of children.
Avoid spraying in eyes.
Store at 25°C (77°F). For optimal results, the canister should be at room temperature when used. Store QVAR Inhalation Aerosol when not being used, so that the product rests on the concave end of the canister with the plastic actuator on top.
Mktd by:Teva Specialty Pharmaceuticals LLCHorsham, PA 19044
Developed and Manufactured by:
3M Drug Delivery SystemsNorthridge, CA 91324
or
3M Health Care, LtdLoughborough, UK
© 2008 Teva Specialty Pharmaceuticals LLCQVAR® is a registered trademark of the IVAX Corporation, a member of the TEVA Group.
634600Rev. 08/08
Principal Display Panel
QVAR® 40mcg
(beclomethasonedipropionate HFA, 40 mcg) INHALATION AEROSOL
For oral inhalation withQVAR® Actuator only
100Metered Inhalations7.3g Net Contents
CFC FREE

QVAR® 80mcg
(beclomethasonedipropionate HFA, 80 mcg) INHALATION AEROSOL
For oral inhalation withQVAR® Actuator only
100Metered Inhalations7.3g Net Contents
CFC FREE
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site




