Boxed Warning
Warning
Go PRO for all pill images
Warning
This fixed combination drug is not indicated for initial therapy of hypertension. Hypertension requires therapy titrated to the individual patient. If the fixed combination represents the dosage so determined, its use may be more convenient in patient management. The treatment of hypertension is not static, but must be reevaluated as conditions in each patient warrant.
Description
RENESE®-R tablets combine polythiazide and reserpine, two antihypertensive agents with complementary properties. Each blue, scored tablet of RENESE-R provides:
Renese (polythiazide). . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.0 mg
Reserpine . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. . . . . . . . .0.25 mg
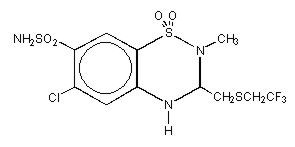
RENESE (polythiazide) is a member of the benzothiazide (thiazide) family of diuretic/antihypertensive agents. It is designated chemically as 2H-1,2,4-Benzothiadiazine-7-sulfonamide,6-chloro-3,4-dihydro-2-methyl-3-[[2,2,2-trifluoroethyl)thio]methyl]-,1,1-dioxide with a molecular formula of C11H13ClF3N3O4S3 and a molecular weight of 439.87.
Polythiazide is a white crystalline substance insoluble in water, but readily soluble in alkaline solution, and has the following structural formula:
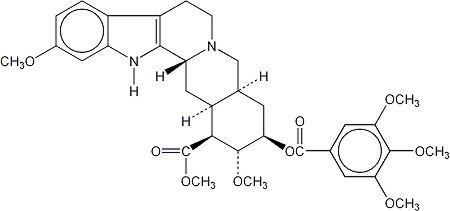
Reserpine, one of the alkaloids of Rauwolfia serpentina, has the following structural formula:
Reserpine – which is administered orally, is insoluble in water, very slightly soluble in ether, l g in about 1800 mL alcohol and about 6 mL chloroform, slightly soluble in benzene, freely soluble in acetic acid.
It has a molecular weight of 608.69 and its molecular formula is C33H40N2O9. Reserpine is chemically designated as Yohimban-16-carboxylic acid, 11,17-dimethoxy-18-[(3,4,5-trimethoxybenzoyl)oxy]-, methyl ester, (3β,16β,17α,18β,20α)-. It is a white or pale buff to slightly yellowish crystalline powder that is insoluble in water.
Inert Ingredients: FD&C Blue No. 1; dibasic calcium phosphate; lactose; magnesium stearate; polyethylene glycol; sodium lauryl sulfate; starch; vanillin.
Clinical Pharmacology
Polythiazide
Renese (polythiazide) alone has demonstrated clinical effectiveness in lowering elevated blood pressure in patients without visible edema as well as in edematous hypertensive patients. Its mechanism of action results in an interference with the renal tubular mechanism of electrolyte reabsorption. At maximal therapeutic dosage all thiazides are approximately equal in their diuretic potency. The mechanism whereby thiazides function in the control of hypertension is unknown. Polythiazide is well absorbed following oral administration with diuresis beginning approximately 2 hours later. Peak human plasma concentrations occur about 5 hours after ingestion. Polythiazide is removed slowly thereafter with a plasma elimination half-life of approximately 27 hours. One-fifth of the drug is recovered unchanged in human urine; the remainder is cleared via feces and as metabolites. Animal studies indicate metabolism occurs by rupture of the thiadiazine ring and loss of the side chain.Reserpine
Reserpine has several complementary actions of benefit to the hypertensive patient, including a calming effect and a slowing of the pulse rate.
Depletion of norepinephrine from tissue receptor sites is thought to be responsible for the decrease in peripheral vascular resistance and subsequent fall in blood pressure. Bradycardia is usually associated with this effect.
The tranquilizing effect of reserpine is apparently due to serotonin and catecholamine depletion in the brain.
Sympathetic inhibition produced by reserpine also may result in vasodilation and increased cutaneous blood flow with resulting flushing, feeling of warmth, or nasal congestion. Increased parasympathomimetic activity may produce increased gastrointestinal motility, increased gastric acid secretion and miosis.
Reserpine is absorbed orally and is widely distributed in body tissues, especially adipose tissue. A study in a small number of normal subjects who received a radioactively labeled 0.25 mg dose of reserpine showed a biphasic half-life of 4.5 hours during the first phase, and 11.3 days during the second phase. The full therapeutic effects of reserpine may not be seen for 2–3 weeks.
Reserpine is extensively metabolized to inactive compounds. It is slowly excreted via the urine and feces.
Reserpine crosses the blood-brain barrier and the placenta, and appears in cord blood.
Since polythiazide reduces or eliminates the sodium and fluid retention frequently associated with hypertension, it enhances the efficacy of reserpine in lowering elevated blood pressure. RENESE-R often has been found to be more effective than equivalent doses of either agent alone. Both the cardiovascular and central nervous system effects may persist following withdrawal of the drug.
Indications And Usage
Hypertension (see box warning).Usage in Pregnancy
The routine use of diuretics in an otherwise healthy woman is inappropriate and exposes mother and fetus to unnecessary hazard. Diuretics do not prevent development of toxemia of pregnancy, and there is no satisfactory evidence that they are useful in the treatment of developed toxemia.
Edema during pregnancy may arise from pathological causes or from the physiologic and mechanical consequences of pregnancy. Thiazides are indicated in pregnancy when edema is due to pathologic causes, just as they are in the absence of pregnancy (however, see Precautions/Pregnancy section). Dependent edema in pregnancy, resulting from restriction of venous return by the expanded uterus, is properly treated through elevation of the lower extremities and use of support hose; use of diuretics to lower intravascular volume in this case is illogical and unnecessary. There is hypervolemia during normal pregnancy which is harmful to neither the fetus nor the mother (in the absence of cardiovascular disease), but which is associated with edema – including generalized edema, in the majority of pregnant women. If this edema produces discomfort, increased recumbency will often provide relief. In rare instances, this edema may cause extreme discomfort which is not relieved by rest. In these cases, a short course of diuretics may provide relief and may be appropriate.
Contraindications
A. Related to polythiazide
- Advanced renal or hepatic failure.
- Hypersensitivity to this or other sulfonamide derivatives.
B. Related to reserpine
- Demonstrated hypersensitivity.
- Patients with a history of mental depression.
- Demonstrated peptic ulcer or ulcerative colitis.
Warnings
Serum electrolyte determinations are especially indicated for patients with severe derangement of metabolic processes, e.g., surgery, vomiting, or parenteral fluid therapy. Electrolyte imbalance may be caused by certain diseases such as cirrhosis, or it may result from drug therapy, such as therapy with corticosteroids. Patients with cirrhosis who are continually receiving RENESE-R should be observed carefully for the development of hepatic precoma or coma. Indications of impending hepatic failure are tremor, confusion, drowsiness, and hepatic fetor.
Thiazides may precipitate kidney failure and uremia in patients with pre-existing renal pathology and impaired renal function.
Available information tends to implicate all oral dosage forms of potassium salts ingested in solid form with or without thiazides in the etiology of nonspecific, small bowel lesions consisting of ulceration with or without stenosis, causing obstruction, hemorrhage and perforation, and frequently requiring surgery. Deaths due to these complications have been reported. All oral dosage forms of potassium salts ingested in solid form should be used only when adequate dietary supplementation is not practical, and should be discontinued immediately if abdominal pain, distention, nausea, vomiting, or gastrointestinal bleeding occur.
RENESE-R does not itself contain enteric-coated potassium.
Electroshock therapy should not be given within one week of cessation of reserpine.
Reserpine may cause mental depression. Recognition of depression may be difficult because this condition may often be disguised by somatic complaints (Masked Depression). The drug should be discontinued at first signs of depression such as despondency, early morning insomnia, loss of appetite, impotence, or self-deprecation. Drug-induced depression may persist for several months after drug withdrawal and may be severe enough to result in suicide.
Precautions
General
Polythiazide
Since all diuretic agents may reduce serum levels of sodium, chloride, and potassium – especially with brisk diuresis or when used concurrently with steroids – patients should be observed regularly for early signs of fluid or electrolyte imbalance, and serum electrolyte studies should be performed periodically. Warning signs of possible electrolyte imbalance, irrespective of cause include fatigue, muscle cramps, gastrointestinal disturbances, lethargy, oliguria, and tachycardia. In extreme cases, hypotension, shock, and coma may develop. Frequently, serum electrolyte levels do not correlate with signs or symptoms of electrolyte imbalance. Unduly restricted salt intake as well as concurrent administration of digitalis may exaggerate metabolic effects of hypokalemia. A favorable ratio of potassium to sodium excretion lessens the possibility of hypokalemia. However, should this occur or be suspected, foods with a high potassium content (bananas, apricots, citrus fruits, prune juice, etc.) should be given. When necessary, oral potassium supplements may be administered. If other antihypertensive agents are used concurrently, lower than usual doses of RENESE-R and of the other agents should be considered.
The antihypertensive effects of the drug may be enhanced in the postsympathectomy patient.Reserpine
Since reserpine may increase gastric acid secretion, it should be used cautiously in patients with a history of peptic ulcer or ulcerative colitis. Extreme caution is needed in patients with a history of mental depression, and reserpine should be discontinued at the first sign of depressive symptoms. Parkinsonism and confusion have been encountered, particularly in psychiatric patients, and constitute an indication for withdrawal of the drug. Caution should be exercised when treating patients with impairment of renal function, as lowered blood pressure may result in further decompensation and embarrassment of function.
Discontinue the drug one to two weeks prior to elective surgery since an unexpected degree of hypotension and bradycardia have been reported in patients receiving anesthetic agents concurrently with reserpine, probably due to a reduced responsiveness to norepinephrine. For emergency surgical procedures vagal blocking agents may be given parenterally to prevent or reverse hypotension and/or bradycardia. Reserpine may cause increased appetite and weight gain in some patients.Information for Patients
Since RENESE-R may increase urination, it is advisable to take it early during the day. If stomach upset occurs, take the drug with food or milk.
Notify your physician if muscle weakness, cramps, nausea, or dizziness occur as these may indicate the loss of too much potassium from your body.
A few people who take this medicine may be more sensitive to sunlight than they are normally. Avoid too much sun or use of a sunlamp until you see how you react.
RENESE-R may cause drowsiness. Make sure you know how you react before driving, using machinery, or performing tasks that require alertness.
Notify your physician if changes in mood or sleep patterns occur. Dizziness or lightheadedness may occur when getting up from a sitting or lying position. Getting up slowly may help. Notify your physician if the problem worsens.Laboratory Tests
Determination of serum electrolytes to detect possible electrolyte imbalance should be performed at appropriate intervals.Drug Interactions
Polythiazide
Thiazides may add to or potentiate the action of other antihypertensive drugs. Potentiation occurs with ganglionic or peripheral adrenergic blocking drugs.
Hypokalemia may be more likely to develop during concomitant use of corticosteroids or ACTH. Diuretic-induced hypokalemia may precipitate digitalis toxicity.
Thiazide drugs may augment the paralyzing actions of tubocurarine, and may decrease the arterial responsiveness to norepinephrine. Extra precautions may be necessary in patients who may require these drugs or their derivatives, as in surgery.
Dosage adjustment of antidiabetic agents is frequently indicated during thiazide administration. Indomethacin may partially antagonize the hypotensive effect of the thiazide diuretics. Generally, do not give lithium with diuretics because they reduce lithiums renal clearance and add a high risk of lithium toxicity.
Quinidine, a weak base, may have its half-life prolonged by concomitant administration of thiazide diuretics which alkalinize the urine.
Sulfonamides may potentiate the action of the thiazide diuretics, possibly by displacement from binding sites on plasma albumin.
Orthostatic hypotension may be aggravated by the use of alcohol, barbiturates, or narcotics with thiazide diuretics.Reserpine
Reserpine should be used cautiously with digitalis or quinidine as the concurrent use may enhance the appearance of arrhythmias.
Additive CNS depressant effects may occur when reserpine is administered concomitantly with other CNS depressants such as barbiturates and alcohol.
Concomitant administration of reserpine and levodopa has been reported to reduce the patient's response to levodopa. Reserpine should be avoided in patients receiving levodopa.
The effects of indirect-acting sympathomimetic amines such as ephedrine may be decreased.
Patients who are receiving monoamine oxidase inhibitors may experience excitation and hypertension when reserpine is added. The combination should be avoided.
Reserpine may add to the pharmacologic effects of beta-adrenergic blocking agents (i.e., CNS depression and cardiovascular effects).Drug/Laboratory Test Interactions
Polythiazide
The thiazides may alter various laboratory test results. These include all electrolytes, particularly potassium, BUN, glucose, and PBI. Thiazides may decrease serum PBI levels without signs of thyroid disturbance.
Like other thiazide diuretics, polythiazide may cause a rise in serum uric acid levels with or without overt symptoms of gout.Reserpine
Chronic administration results in a decrease in urinary catecholamines and vanilmandelic acid excretion.
Interference with colorimetric assay procedures for the determination of urinary 17-hydroxycorticosteroids by the Glenn-Nelson technique and 17-ketosteroids by the Holtorff Koch modification of the Zimmerman reaction have been reported.Animal Tumorigenicity
Although long-term studies in animals have not been conducted with RENESE-R, rodent studies have shown that reserpine is an animal tumorigen, causing an increased incidence of mammary fibroadenomas in female mice, malignant tumors of the seminal vesicles in male mice, and malignant adrenal medullary tumors in male rats. These findings arose in 2 year studies in which the drug was administered in the feed at concentrations of 5 and 10 ppm—about 100 to 300 times the usual human dose. The breast neoplasms are thought to be related to reserpine's prolactin-elevating effect.
Several other prolactin-elevating drugs have also been associated with an increased incidence of mammary neoplasia in rodents.
The extent to which these findings indicate a risk to humans is uncertain. Tissue culture experiments show that about one-third of human breast tumors are prolactin-dependent in vitro, a factor of considerable importance if the use of the drug is contemplated in a patient with previously detected breast cancer. The possibility of an increased risk of breast cancer in reserpine users has been studied extensively; however, no firm conclusion has emerged. Although a few epidemiologic studies have suggested a slightly increased risk (less than twofold in all studies except one) in women who have used reserpine, other studies of generally similar design have not confirmed this. Epidemiologic studies conducted using other drugs (neuroleptic agents) that, like reserpine, increase prolactin levels and therefore would be considered rodent mammary carcinogens, have not shown an association between chronic administration of the drug and human mammary tumorigenesis. While long-term clinical observation has not suggested such an association, the available evidence is considered too limited to be conclusive at this time. An association of reserpine intake with pheochromocytoma or tumors of the seminal vesicles has not been explored.Pregnancy
Teratogenic Effects
Pregnancy Category C
Animal reproduction studies have not been conducted with RENESE-R. There are no adequate and well controlled studies in pregnant women. It is also not known whether RENESE-R can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. RENESE-R should be given to a pregnant woman only if clearly needed.Nonteratogenic Effects
Thiazides cross the placental barrier and appear in cord blood. When polythiazide and reserpine are used in women of childbearing age, the potential benefits of this drug combination should be weighed against the possible hazards to the fetus. The hazards include fetal or neonatal jaundice, thrombocytopenia, and possible other adverse reactions which have occurred in the adult.Nursing Mothers
Thiazides and reserpine appear in breast milk. Because of the potential for serious adverse reactions in nursing infants from RENESE-R, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.Pediatric Use
Safety and effectiveness in children have not been established.
Adverse Reactions
The following adverse reactions have been observed, but there is not enough systemic collection of data to support an estimate of their frequency.Polythiazide
The most common reactions associated with polythiazide therapy are weakness and dizziness, but seldom require cessation of therapy. Weakness is reported in less than 3% of patients receiving the drug, and dizziness is reported in less than 2% of patients. These can be overcome by reducing the dose or taking measures to improve electrolytic balance.
Other reactions, reported to occur in less than 1% of patients, are:
Gastrointestinal: nausea, gastrointestinal disturbances, reversible cholestatic jaundice, necrotizing angiitis, pancreatitis.
Dermatological: maculopapular skin rash, photosensitivity.
Central Nervous System: vertigo, paresthesia, fatigue, headache.
Cardiovascular: orthostatic hypotension.
EENT: xanthopsia.
Hematological: Leukopenia (neutropenia), agranulocytosis, and aplastic anemia have been reported with the older thiazides but not with the newer compounds such as polythiazide. Purpura (with or without thrombocytopenia) has been reported with polythiazide.Reserpine
The most common reactions associated with reserpine therapy, dizziness and drowsiness, are reported in less than 2% of patients. Reactions to reserpine are usually reversible and disappear when the drug is discontinued.
Other reactions, occurring in less than 1% of patients on reserpine are:
Gastrointestinal: hypersecretion, nausea, vomiting, diarrhea, anorexia, dry mouth.
Dermatological: rash, pruritus, purpura.
Central Nervous System: depression, nervousness, paradoxical anxiety, nightmares, headache, and rare Parkinsonian syndrome to CNS sensitization manifested by deafness, glaucoma, uveitis, and optic atrophy.
Cardiovascular: angina-like symptoms, arrhythmias particularly when used concurrently with digitalis or quinidine, flushing of the skin, and bradycardia.
EENT: nasal congestion, miosis.
Sexual Difficulties: impotence or decreased libido.
Overdosage
One case of RENESE-R overdosage is reported after ingestion of an unknown number of tablets. Electrolyte replacement therapy was successful in treating the symptoms.Polythiazide
An overdose of Renese may cause electrolyte imbalance, manifested by fatigue, muscle weakness, cramps, gastrointestinal disturbances, lethargy, tachycardia, and/or other arrhythmias, and hypotension.
Should overdosage with Renese occur, electrolyte balance and adequate hydration should be maintained. Gastric lavage is recommended followed by supportive treatment. Where necessary, this may include intravenous dextrose and saline with potassium and other electrolyte therapy, administered with caution as indicated by laboratory testing at appropriate intervals.Reserpine
Signs of overdosage include CNS depression ranging from drowsiness to coma, bradycardia and hypotension, respiratory depression, hypothermia, diarrhea, vomiting, mental depression, skin flushing, miosis, and extrapyramidal signs such as stiffness and tremors. Emesis or gastric lavage to remove unabsorbed drug is of benefit in conscious patients, even if several hours have elapsed since ingestion. Treatment is symptomatic and supportive.
Parasympathomimetic side effects usually can be controlled with small doses of atropine or other anticholinergics. Evidence of motor dysfunction often can be controlled by drugs useful for parkinsonism. Avoid vasopressor drugs (except in cases of extreme hypotension) and rapid intravenous infusions because of the uncertain cardiac status.
It is not known whether dialysis would be of benefit in treating cases of overdosage of RENESE-R.
Dosage And Administration
As determined by individual titration (see box warning).
Initial dosages of the combination should conform to those dosages of the individual components established during titration.
Maintenance dosages range from ½ tablet to 2 tablets daily. Dosage of other antihypertensive agents, particularly ganglionic blockers, that are used concomitantly should be reduced.
How Supplied
RENESE-R tablets (2 mg polythiazide–0.25 mg reserpine) are available as blue scored tablets,
tablet code 446, in bottles of 100 (NDC 0069-4460 -66) and 1,000
(NDC-0069-4460-82). RENESE-R should be stored at room temperature. Dispense in tight, light-resistant container.
Rx only
Distributed by:
Pfizer Labs
Division of Pfizer, Inc, NY, NY 10017
69-1200-00-3
September 1995
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site