RISPERDAL (risperidone 3 mg) Dailymed
Generic: risperidone is used for the treatment of Autistic Disorder Bipolar Disorder Dementia Tourette Syndrome Schizophrenia
IMPRINT: JANSSEN R 1
SHAPE: oval
COLOR: white
All Imprints
risperdal (risperidone) tablet risperdal m-tab (risperidone) tablet, orally disintegrating risperdal (risperidone) solution - janssen r 1 oval white
risperidone 2 mg - r2 square orange
risperidone 1 mg - r1 janssen oval white
risperidone 0.5 mg - ris 0 5 janssen oval brown
risperidone 0.25 mg - ris 0 25 janssen oval yellow
risperidone 2 mg - r2 janssen oval orange
risperidone 3 mg - r3 janssen oval yellow
Boxed Warning
Warning: Increased Mortality In Elderly Patients With Dementia-related Psychosis
Go PRO for all pill images
Warning: Increased Mortality In Elderly Patients With Dementia-related Psychosis
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. RISPERDAL® is not approved for the treatment of patients with dementia-related psychosis. [seeWarnings and Precautions (5.1)]
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
See full prescribing information for complete boxed warning.
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. RISPERDAL® is not approved for use in patients with dementia-related psychosis. (5.1 )
Recent Major Changes Section
Dosage and Administration ( 2.6 )3/2022
1 Indications And Usage
RISPERDAL® is an atypical antipsychotic indicated for:
- Treatment of schizophrenia (
1.1 )- As monotherapy or adjunctive therapy with lithium or valproate, for the treatment of acute manic or mixed episodes associated with Bipolar I Disorder (
1.2 )- Treatment of irritability associated with autistic disorder (
1.3 )1.1Schizophrenia
RISPERDAL® (risperidone) is indicated for the treatment of schizophrenia. Efficacy was established in 4 short-term trials in adults, 2 short-term trials in adolescents (ages 13 to 17 years), and one long-term maintenance trial in adults [see Clinical Studies (14.1)].
1.2Bipolar Mania
Monotherapy
RISPERDAL® is indicated for the treatment of acute manic or mixed episodes associated with Bipolar I Disorder. Efficacy was established in 2 short-term trials in adults and one short-term trial in children and adolescents (ages 10 to 17 years) [see Clinical Studies (14.2)].
Adjunctive Therapy
RISPERDAL® adjunctive therapy with lithium or valproate is indicated for the treatment of acute manic or mixed episodes associated with Bipolar I Disorder. Efficacy was established in one short-term trial in adults [see Clinical Studies (14.3)].
1.3Irritability Associated with Autistic Disorder
RISPERDAL® is indicated for the treatment of irritability associated with autistic disorder, including symptoms of aggression towards others, deliberate self-injuriousness, temper tantrums, and quickly changing moods. Efficacy was established in 3 short-term trials in children and adolescents (ages 5 to 17 years) [see Clinical Studies (14.4)].
2 Dosage And Administration
Table 1. Recommended Daily Dosage by Indication Initial Dose Titration (Increments) Target Dose Effective Dose Range Schizophrenia: adults (2.1) 2 mg 1 to 2 mg 4 to 8 mg 4 to 16 mg Schizophrenia: adolescents(2.2) 0.5 mg 0.5 to 1 mg 3 mg 1 to 6 mg Bipolar mania: adults(2.2) 2 to 3 mg 1 mg 1 to 6 mg 1 to 6 mg Bipolar mania: children and adolescents(2.2) 0.5 mg 0.5 to 1 mg 1 to 2.5 mg 1 to 6 mg Irritability in autistic disorder(2.3) 0.25 mgCan increase to 0.5 mg by Day 4:(body weight less than 20 kg) 0.5 mgCan increase to 1 mg by Day 4:(body weight greater than or equal to 20 kg) After Day 4, at intervals of > 2 weeks:0.25 mg(body weight less than 20 kg) 0.5 mg(body weight greater than or equal to 20 kg) 0.5 mg:(body weight less than 20 kg) 1 mg:(body weight greater than or equal to 20 kg) 0.5 to 3 mg
Severe Renal and Hepatic Impairment in Adults: use a lower starting dose of 0.5 mg twice daily. May increase to dosages above 1.5 mg twice daily at intervals of one week or longer.
- Recommended daily dosage:
Initial Dose Target Dose Effective Dose Range Schizophrenia: adults ( 2.1 )2 mg 4 to 8 mg 4 to 16 mg Schizophrenia: adolescents ( 2.1 )0.5 mg 3 mg 1 to 6 mg Bipolar mania: adults ( 2.2 )2 to 3 mg 1 to 6 mg 1 to 6 mg Bipolar mania: in children and adolescents ( 2.2 )0.5 mg 1 to 2.5 mg 1 to 6 mg Irritability associated with autistic disorder ( 2.3 )0.25 mg(Weight < 20 kg) 0.5 mg(Weight ≥20 kg) 0.5 mg(<20 kg) 1 mg(≥20 kg) 0.5 to 3 mg
- Severe Renal or Hepatic Impairment in Adults: Use a lower starting dose of 0.5 mg twice daily. May increase to dosages above 1.5 mg twice daily at intervals of at least one week. (
2.4 )- Oral Solution: Can be administered directly from calibrated oral dosing syringe or mixed with beverage (water, coffee, orange juice, or low-fat milk). (
2.6 )- M-TAB Orally Disintegrating Tablets: Open the buler only when ready to administer, and immediately place tablet on the tongue. Can be swallowed with or without liquid. (
2.7 )2.1 Schizophrenia
Adults
Usual Initial Dose
RISPERDAL® can be administered once or twice daily. Initial dosing is 2 mg per day. May increase the dose at intervals of 24 hours or greater, in increments of 1 to 2 mg per day, as tolerated, to a recommended dose of 4 to 8 mg per day. In some patients, slower titration may be appropriate. Efficacy has been demonstrated in a range of 4 mg to 16 mg per day. However, doses above 6 mg per day for twice daily dosing were not demonstrated to be more efficacious than lower doses, were associated with more extrapyramidal symptoms and other adverse effects, and are generally not recommended. In a single study supporting once-daily dosing, the efficacy results were generally stronger for 8 mg than for 4 mg. The safety of doses above 16 mg per day has not been evaluated in clinical trials [seeClinical Studies (14.1)].
Adolescents
The initial dose is 0.5 mg once daily, administered as a single-daily dose in the morning or evening. The dose may be adjusted at intervals of 24 hours or greater, in increments of 0.5 mg or 1 mg per day, as tolerated, to a recommended dose of 3 mg per day. Although efficacy has been demonstrated in studies of adolescent patients with schizophrenia at doses between 1 mg to 6 mg per day, no additional benefit was observed above 3 mg per day, and higher doses were associated with more adverse events. Doses higher than 6 mg per day have not been studied.
Patients experiencing persistent somnolence may benefit from administering half the daily dose twice daily.
Maintenance Therapy
While it is unknown how long a patient with schizophrenia should remain on RISPERDAL®, the effectiveness of RISPERDAL® 2 mg per day to 8 mg per day at delaying relapse was demonstrated in a controlled trial in adult patients who had been clinically stable for at least 4 weeks and were then followed for a period of 1 to 2 years [see Clinical Studies (14.1)]. Both adult and adolescent patients who respond acutely should generally be maintained on their effective dose beyond the acute episode. Patients should be periodically reassessed to determine the need for maintenance treatment.
Reinitiation of Treatment in Patients Previously Discontinued
Although there are no data to specifically address reinitiation of treatment, it is recommended that after an interval off RISPERDAL®, the initial titration schedule should be followed.
Switching From Other Antipsychotics
There are no systematically collected data to specifically address switching schizophrenic patients from other antipsychotics to RISPERDAL®, or treating patients with concomitant antipsychotics.
2.2Bipolar Mania
Usual Dose
Adults
The initial dose range is 2 mg to 3 mg per day. The dose may be adjusted at intervals of 24 hours or greater, in increments of 1 mg per day. The effective dose range is 1 mg to 6 mg per day, as studied in the short-term, placebo-controlled trials. In these trials, short-term (3 week) anti-manic efficacy was demonstrated in a flexible dosage range of 1 mg to 6 mg per day [see Clinical Studies (14.2 , 14.3)]. RISPERDAL® doses higher than 6 mg per day were not studied.
Pediatrics
The initial dose is 0.5 mg once daily, administered as a single-daily dose in the morning or evening. The dose may be adjusted at intervals of 24 hours or greater, in increments of 0.5 mg or 1 mg per day, as tolerated, to the recommended target dose of 1 mg to 2.5 mg per day. Although efficacy has been demonstrated in studies of pediatric patients with bipolar mania at doses between 0.5 mg and 6 mg per day, no additional benefit was observed above 2.5 mg per day, and higher doses were associated with more adverse events. Doses higher than 6 mg per day have not been studied.
Patients experiencing persistent somnolence may benefit from administering half the daily dose twice daily.
Maintenance Therapy
There is no body of evidence available from controlled trials to guide a clinician in the longer-term management of a patient who improves during treatment of an acute manic episode with RISPERDAL®. While it is generally agreed that pharmacological treatment beyond an acute response in mania is desirable, both for maintenance of the initial response and for prevention of new manic episodes, there are no systematically obtained data to support the use of RISPERDAL® in such longer-term treatment (i.e., beyond 3 weeks). The physician who elects to use RISPERDAL® for extended periods should periodically re-evaluate the long-term risks and benefits of the drug for the individual patient.
2.3Irritability Associated with Autistic Disorder Pediatrics (Children and Adolescents)
The dosage of RISPERDAL® should be individualized according to the response and tolerability of the patient. The total daily dose of RISPERDAL® can be administered once daily, or half the total daily dose can be administered twice daily.
For patients with body weight less than 20 kg, initiate dosing at 0.25 mg per day. For patients with body weight greater than or equal to 20 kg, initiate dosing at 0.5 mg per day. After a minimum of four days, the dose may be increased to the recommended dose of 0.5 mg per day for patients less than 20 kg and 1.0 mg per day for patients greater than or equal to 20 kg. Maintain this dose for a minimum of 14 days. In patients not achieving sufficient clinical response, the dose may be increased at intervals of 2 weeks or greater, in increments of 0.25 mg per day for patients less than 20 kg, or increments of 0.5 mg per day for patients greater than or equal to 20 kg. The effective dose range is 0.5 mg to 3 mg per day. No dosing data are available for children who weigh less than 15 kg.
Once sufficient clinical response has been achieved and maintained, consider gradually lowering the dose to achieve the optimal balance of efficacy and safety. The physician who elects to use RISPERDAL® for extended periods should periodically re-evaluate the long-term risks and benefits of the drug for the individual patient.
Patients experiencing persistent somnolence may benefit from a once-daily dose administered at bedtime or administering half the daily dose twice daily, or a reduction of the dose.
2.4Dosing in Patients with Severe Renal or Hepatic Impairment
For patients with severe renal impairment (Clcr< 30 mL/min) or hepatic impairment (10–15 points on Child Pugh System), the initial starting dose is 0.5 mg twice daily. The dose may be increased in increments of 0.5 mg or less, administered twice daily. For doses above 1.5 mg twice daily, increase in intervals of one week or greater [seeUse in Specific Populations (8.6 and8.7)].
2.5Dose Adjustments for Specific Drug Interactions
When RISPERDAL® is co-administered with enzyme inducers (e.g., carbamazepine), the dose of RISPERDAL® should be increased up to double the patient's usual dose. It may be necessary to decrease the RISPERDAL® dose when enzyme inducers such as carbamazepine are discontinued [see Drug Interactions (7.1)]. Similar effect may be expected with co-administration of RISPERDAL® with other enzyme inducers (e.g., phenytoin, rifampin, and phenobarbital).
When fluoxetine or paroxetine is co-administered with RISPERDAL®, the dose of RISPERDAL® should be reduced. The RISPERDAL® dose should not exceed 8 mg per day in adults when co-administered with these drugs. When initiating therapy, RISPERDAL® should be titrated slowly. It may be necessary to increase the RISPERDAL® dose when enzyme inhibitors such as fluoxetine or paroxetine are discontinued [see Drug Interactions (7.1)].
2.6 Administration of RISPERDAL Oral Solution
RISPERDAL® Oral Solution can be administered directly from the calibrated oral dosing syringe, or can be mixed with a beverage prior to administration. RISPERDAL® Oral Solution is compatible in the following beverages: water, coffee, orange juice, and low-fat milk; it is NOT compatible with either cola or tea.
2.7 Directions for Use of RISPERDAL M-TAB Orally Disintegrating Tablets
Tablet Accessing
RISPERDAL® M-TAB® Orally Disintegrating Tablets 0.5 mg, 1 mg, and 2 mg
RISPERDAL® M-TAB® Orally Disintegrating Tablets 0.5 mg, 1 mg, and 2 mg are supplied in buler packs of 4 tablets each.
Do not open the buler until ready to administer. For single tablet removal, separate one of the four buler units by tearing apart at the perforations. Bend the corner where indicated. Peel back foil to expose the tablet. DO NOT push the tablet through the foil because this could damage the tablet.
RISPERDAL® M-TAB® Orally Disintegrating Tablets 3 mg and 4 mg
RISPERDAL® M-TAB® Orally Disintegrating Tablets 3 mg and 4 mg are supplied in a child-resistant pouch containing a buler with 1 tablet each.
The child-resistant pouch should be torn open at the notch to access the buler. Do not open the buler until ready to administer. Peel back foil from the side to expose the tablet. DO NOT push the tablet through the foil, because this could damage the tablet.
Tablet Administration
Using dry hands, remove the tablet from the buler unit and immediately place the entire RISPERDAL® M-TAB® Orally Disintegrating Tablet on the tongue. The RISPERDAL® M-TAB® Orally Disintegrating Tablet should be consumed immediately, as the tablet cannot be stored once removed from the buler unit. RISPERDAL® M-TAB® Orally Disintegrating Tablets disintegrate in the mouth within seconds and can be swallowed subsequently with or without liquid. Patients should not attempt to split or to chew the tablet.
3 Dosage Forms And Strengths
RISPERDAL® Tablets are available in the following strengths and colors: 0.5 mg (red-brown), 1 mg (white), 2 mg (orange), 3 mg (yellow), and 4 mg (green). All are capsule shaped, and imprinted with "JANSSEN" on one side and either "Ris 0.5", "R1", "R2", "R3", or "R4" on the other side according to their respective strengths.
RISPERDAL® Oral Solution is available in a 1 mg/mL strength.
RISPERDAL® M-TAB® Orally Disintegrating Tablets are available in the following strengths, colors, and shapes: 0.5 mg (light coral, round), 1 mg (light coral, square), 2 mg (coral, square), 3 mg (coral, round), and 4 mg (coral, round). All are biconvex and etched on one side with "R0.5", "R1", "R2", "R3", or "R4" according to their respective strengths.
- Tablets: 0.5 mg, 1 mg, 2 mg, 3 mg, and 4 mg (
3 )- Oral solution: 1 mg per mL (
3 )- Orally disintegrating tablets: 0.5 mg, 1 mg, 2 mg, 3 mg, and 4 mg (
3 )
4 Contraindications
RISPERDAL® is contraindicated in patients with a known hypersensitivity to either risperidone or paliperidone, or to any of the excipients in the RISPERDAL® formulation. Hypersensitivity reactions, including anaphylactic reactions and angioedema, have been reported in patients treated with risperidone and in patients treated with paliperidone. Paliperidone is a metabolite of risperidone.
- Known hypersensitivity to risperidone, paliperidone, or to any excipients in RISPERDAL®. (
4 )
5 Warnings And Precautions
- Cerebrovascular events, including stroke, in elderly patients with dementia-related psychosis: RISPERDAL® is not approved for use in patients with dementia-related psychosis. (
5.2 )- Neuroleptic Malignant Syndrome: Manage with immediate discontinuation of RISPERDAL® and close monitoring. (
5.3 )- Tardive dyskinesia: Consider discontinuing RISPERDAL® if clinically indicated. (
5.4 )- Metabolic Changes: Atypical antipsychotic drugs have been associated with metabolic changes that may increase cardiovascular/ cerebrovascular risk. These metabolic changes include hyperglycemia, dyslipidemia, and weight gain. (
5.5 )
- Hyperglycemia and Diabetes Mellitus: Monitor patients for symptoms of hyperglycemia including polydipsia, polyuria, polyphagia, and weakness. Monitor glucose regularly in patients with diabetes or at risk for diabetes. (
5.5 )- Dyslipidemia: Undesirable alterations have been observed in patients treated with atypical antipsychotics. (
5.5 )- Weight Gain: Significant weight gain has been reported. Monitor weight gain. (
5.5 )- Hyperprolactinemia: Prolactin elevations occur and persist during chronic administration. (
5.6 )- Orthostatic hypotension: For patients at risk, consider a lower starting dose and slower titration. (
5.7 )- Leukopenia, Neutropenia, and Agranulocytosis: Perform complete blood counts in patients with a history of clinically significant low white blood cell count (WBC). Consider discontinuing RISPERDAL® if a clinically significant decline in WBC occurs in the absence of other causative factors. (
5.9 )- Potential for cognitive and motor impairment: Use caution when operating machinery. (
5.10 )- Seizures: Use cautiously in patients with a history of seizures or with conditions that lower the seizure threshold. (
5.11 )5.1Increased Mortality in Elderly Patients with Dementia-Related Psychosis
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of 17 placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear.
In two of four placebo-controlled trials in elderly patients with dementia-related psychosis, a higher incidence of mortality was observed in patients treated with furosemide plus RISPERDAL® when compared to patients treated with RISPERDAL® alone or with placebo plus furosemide. No pathological mechanism has been identified to explain this finding, and no consistent pattern for cause of death was observed.
RISPERDAL® (risperidone) is not approved for the treatment of dementia-related psychosis [seeBoxed Warning].
5.2Cerebrovascular Adverse Reactions, Including Stroke, in Elderly Patients with Dementia-Related Psychosis
Cerebrovascular adverse reactions (e.g., stroke, transient ischemic attack), including fatalities, were reported in patients (mean age 85 years; range 73–97) in trials of risperidone in elderly patients with dementia-related psychosis. In placebo-controlled trials, there was a significantly higher incidence of cerebrovascular adverse events in patients treated with risperidone compared to patients treated with placebo. RISPERDAL® is not approved for the treatment of patients with dementia-related psychosis. [see Boxed Warning and Warnings and Precautions (5.1)].
5.3Neuroleptic Malignant Syndrome
Neuroleptic Malignant Syndrome (NMS), a potentially fatal symptom complex, has been reported in association with antipsychotic drugs. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status including delirium, and autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmia). Additional signs may include elevated creatine phosphokinase, myoglobinuria (rhabdomyolysis), and acute renal failure.
If NMS is suspected, immediately discontinue RISPERDAL® and provide symptomatic treatment and monitoring.
5.4Tardive Dyskinesia
Tardive dyskinesia, a syndrome consisting of potentially irreversible, involuntary, dyskinetic movements, may develop in patients treated with antipsychotic drugs. Although the prevalence of the syndrome appears to be highest among the elderly, especially elderly women, it is impossible to predict which patients will develop the syndrome. Whether antipsychotic drug products differ in their potential to cause tardive dyskinesia is unknown.
The risk of developing tardive dyskinesia and the likelihood that it will become irreversible increase with the duration of treatment and the cumulative dose. The syndrome can develop after relatively brief treatment periods, even at low doses. It may also occur after discontinuation of treatment.
Tardive dyskinesia may remit, partially or completely, if antipsychotic treatment is discontinued. Antipsychotic treatment, itself, however, may suppress (or partially suppress) the signs and symptoms of the syndrome, possibly masking the underlying process. The effect that symptomatic suppression has upon the long-term course of the syndrome is unknown.
Given these considerations, RISPERDAL® should be prescribed in a manner that is most likely to minimize the occurrence of tardive dyskinesia. Chronic antipsychotic treatment should generally be reserved for patients: (1) who suffer from a chronic illness that is known to respond to antipsychotic drugs, and (2) for whom alternative, equally effective, but potentially less harmful treatments are not available or appropriate. In patients who do require chronic treatment, use the lowest dose and the shortest duration of treatment producing a satisfactory clinical response. Periodically reassess the need for continued treatment.
If signs and symptoms of tardive dyskinesia appear in a patient on RISPERDAL®, drug discontinuation should be considered. However, some patients may require treatment with RISPERDAL® despite the presence of the syndrome.
5.5Metabolic Changes
Atypical antipsychotic drugs have been associated with metabolic changes that may increase cardiovascular/cerebrovascular risk. These metabolic changes include hyperglycemia, dyslipidemia, and body weight gain. While all of the drugs in the class have been shown to produce some metabolic changes, each drug has its own specific risk profile.
Hyperglycemia and Diabetes Mellitus
Hyperglycemia and diabetes mellitus, in some cases extreme and associated with ketoacidosis or hyperosmolar coma or death, have been reported in patients treated with atypical antipsychotics including RISPERDAL®. Assessment of the relationship between atypical antipsychotic use and glucose abnormalities is complicated by the possibility of an increased background risk of diabetes mellitus in patients with schizophrenia and the increasing incidence of diabetes mellitus in the general population. Given these confounders, the relationship between atypical antipsychotic use and hyperglycemia-related adverse events is not completely understood. However, epidemiological studies suggest an increased risk of treatment-emergent hyperglycemia-related adverse events in patients treated with the atypical antipsychotics. Precise risk estimates for hyperglycemia-related adverse events in patients treated with atypical antipsychotics are not available.
Patients with an established diagnosis of diabetes mellitus who are started on atypical antipsychotics, including RISPERDAL®, should be monitored regularly for worsening of glucose control. Patients with risk factors for diabetes mellitus (e.g., obesity, family history of diabetes) who are starting treatment with atypical antipsychotics, including RISPERDAL®, should undergo fasting blood glucose testing at the beginning of treatment and periodically during treatment. Any patient treated with atypical antipsychotics, including RISPERDAL®, should be monitored for symptoms of hyperglycemia including polydipsia, polyuria, polyphagia, and weakness. Patients who develop symptoms of hyperglycemia during treatment with atypical antipsychotics, including RISPERDAL®, should undergo fasting blood glucose testing. In some cases, hyperglycemia has resolved when the atypical antipsychotic, including RISPERDAL®, was discontinued; however, some patients required continuation of anti-diabetic treatment despite discontinuation of RISPERDAL®.
Pooled data from three double-blind, placebo-controlled schizophrenia studies and four double-blind, placebo-controlled bipolar monotherapy studies are presented in Table 2.
Table 2. Change in Random Glucose from Seven Placebo-Controlled, 3- to 8-Week, Fixed- or Flexible-Dose Studies in Adult Subjects with Schizophrenia or Bipolar Mania RISPERDAL® Placebo 1–8 mg/day >8–16 mg/day Mean change from baseline (mg/dL) n=555 n=748 n=164 Serum Glucose -1.4 0.8 0.6 Proportion of patients with shifts Serum Glucose(<140 mg/dL to ≥200 mg/dL) 0.6%(3/525) 0.4%(3/702) 0%(0/158)
In longer-term, controlled and uncontrolled studies, RISPERDAL® was associated with a mean change in glucose of +2.8 mg/dL at Week 24 (n=151) and +4.1 mg/dL at Week 48 (n=50).
Data from the placebo-controlled 3- to 6-week study in children and adolescents with schizophrenia (13–17 years of age), bipolar mania (10–17 years of age), or autistic disorder (5 to 17 years of age) are presented in Table 3.
Table 3. Change in Fasting Glucose from Three Placebo-Controlled, 3- to 6-Week, Fixed-Dose Studies in Children and Adolescents with Schizophrenia (13–17 years of age), Bipolar Mania (10–17 years of age), or Autistic Disorder (5 to 17 years of age) RISPERDAL® Placebo 0.5–6 mg/day Mean change from baseline (mg/dL) n=76 n=135 Serum Glucose -1.3 2.6 Proportion of patients with shifts Serum Glucose(<100 mg/dL to ≥126 mg/dL) 0%(0/64) 0.8%(1/120)
In longer-term, uncontrolled, open-label extension pediatric studies, RISPERDAL® was associated with a mean change in fasting glucose of +5.2 mg/dL at Week 24 (n=119).
Dyslipidemia
Undesirable alterations in lipids have been observed in patients treated with atypical antipsychotics.
Pooled data from 7 placebo-controlled, 3- to 8- week, fixed- or flexible-dose studies in adult subjects with schizophrenia or bipolar mania are presented in Table 4.
Table 4. Change in Random Lipids from Seven Placebo-Controlled, 3- to 8-Week, Fixed- or Flexible-Dose Studies in Adult Subjects with Schizophrenia or Bipolar Mania RISPERDAL® Placebo 1–8 mg/day >8–16 mg/day Mean change from baseline (mg/dL) Cholesterol n=559 n=742 n=156 Change from baseline 0.6 6.9 1.8 Triglycerides n=183 n=307 n=123 Change from baseline -17.4 -4.9 -8.3 Proportion of patients With Shifts Cholesterol (<200 mg/dL to ≥240 mg/dL) 2.7% 4.3% 6.3% (10/368) (22/516) (6/96) Triglycerides (<500 mg/dL to ≥500 mg/dL) 1.1%(2/180) 2.7%(8/301) 2.5%(3/121)
In longer-term, controlled and uncontrolled studies, RISPERDAL® was associated with a mean change in (a) non-fasting cholesterol of +4.4 mg/dL at Week 24 (n=231) and +5.5 mg/dL at Week 48 (n=86); and (b) non-fasting triglycerides of +19.9 mg/dL at Week 24 (n=52).
Pooled data from 3 placebo-controlled, 3- to 6-week, fixed-dose studies in children and adolescents with schizophrenia (13–17 years of age), bipolar mania (10–17 years of age), or autistic disorder (5–17 years of age) are presented in Table 5.
Table 5. Change in Fasting Lipids from Three Placebo-Controlled, 3- to 6-Week, Fixed-Dose Studies in Children and Adolescents with Schizophrenia (13–17 Years of Age), Bipolar Mania (10–17 Years of Age), or Autistic Disorder (5 to 17 Years of Age) RISPERDAL® Placebo 0.5–6 mg/day Mean change from baseline (mg/dL) Cholesterol n=74 n=133 Change from baseline 0.3 -0.3 LDL n=22 n=22 Change from baseline 3.7 0.5 HDL n=22 n=22 Change from baseline 1.6 -1.9 Triglycerides n=77 n=138 Change from baseline -9.0 -2.6 Proportion of patients with shifts Cholesterol (<170 mg/dL to ≥200 mg/dL) 2.4%(1/42) 3.8%(3/80) LDL (<110 mg/dL to ≥130 mg/dL) 0%(0/16) 0%(0/16) HDL (≥40 mg/dL to <40 mg/dL) 0%(0/19) 10%(2/20) Triglycerides (<150 mg/dL to ≥200 mg/dL) 1.5%(1/65) 7.1%(8/113)
In longer-term, uncontrolled, open-label extension pediatric studies, RISPERDAL® was associated with a mean change in (a) fasting cholesterol of +2.1 mg/dL at Week 24 (n=114); (b) fasting LDL of -0.2 mg/dL at Week 24 (n=103); (c) fasting HDL of +0.4 mg/dL at Week 24 (n=103); and (d) fasting triglycerides of +6.8 mg/dL at Week 24 (n=120).
Weight Gain
Weight gain has been observed with atypical antipsychotic use. Clinical monitoring of weight is recommended.
Data on mean changes in body weight and the proportion of subjects meeting a weight gain criterion of 7% or greater of body weight from 7 placebo-controlled, 3- to 8- week, fixed- or flexible-dose studies in adult subjects with schizophrenia or bipolar mania are presented in Table 6.
Table 6. Mean Change in Body Weight (kg) and the Proportion of Subjects with ≥7% Gain in Body Weight From Seven Placebo-Controlled, 3- to 8-Week, Fixed- or Flexible-Dose Studies in Adult Subjects With Schizophrenia or Bipolar Mania RISPERDAL® Placebo(n=597) 1–8 mg/day(n=769) >8–16 mg/day(n=158) Weight (kg) Change from baseline -0.3 0.7 2.2 Weight Gain ≥7% increase from baseline 2.9% 8.7% 20.9%
In longer-term, controlled and uncontrolled studies, RISPERDAL® was associated with a mean change in weight of +4.3 kg at Week 24 (n=395) and +5.3 kg at Week 48 (n=203).
Data on mean changes in body weight and the proportion of subjects meeting the criterion of ≥7% gain in body weight from nine placebo-controlled, 3- to 8-week, fixed-dose studies in children and adolescents with schizophrenia (13–17 years of age), bipolar mania (10–17 years of age), autistic disorder (5–17 years of age), or other psychiatric disorders (5–17 years of age) are presented in Table 7.
Table 7. Mean Change in Body Weight (kg) and the Proportion of Subjects With ≥7% Gain in Body Weight From Nine Placebo-Controlled, 3- to 8-Week, Fixed-Dose Studies in Children and Adolescents With Schizophrenia (13–17 Years of Age), Bipolar Mania (10–17 Years of Age), Autistic Disorder (5 to 17 Years of Age) or Other Psychiatric Disorders (5–17 Years of Age) Placebo(n=375) RISPERDAL® 0.5–6 mg/day(n=448) Weight (kg) Change from baseline 0.6 2.0 Weight Gain ≥7% increase from baseline 6.9% 32.6%
In longer-term, uncontrolled, open-label extension pediatric studies, RISPERDAL® was associated with a mean change in weight of +5.5 kg at Week 24 (n=748) and +8.0 kg at Week 48 (n=242).
In a long-term, open-label extension study in adolescent patients with schizophrenia, weight increase was reported as a treatment-emergent adverse event in 14% of patients. In 103 adolescent patients with schizophrenia, a mean increase of 9.0 kg was observed after 8 months of RISPERDAL® treatment. The majority of that increase was observed within the first 6 months. The average percentiles at baseline and 8 months, respectively, were 56 and 72 for weight, 55 and 58 for height, and 51 and 71 for body mass index.
In long-term, open-label trials (studies in patients with autistic disorder or other psychiatric disorders), a mean increase of 7.5 kg after 12 months of RISPERDAL® treatment was observed, which was higher than the expected normal weight gain (approximately 3 to 3.5 kg per year adjusted for age, based on Centers for Disease Control and Prevention normative data). The majority of that increase occurred within the first 6 months of exposure to RISPERDAL®. The average percentiles at baseline and 12 months, respectively, were 49 and 60 for weight, 48 and 53 for height, and 50 and 62 for body mass index.
In one 3-week, placebo-controlled trial in children and adolescent patients with acute manic or mixed episodes of bipolar I disorder, increases in body weight were higher in the RISPERDAL® groups than the placebo group, but not dose related (1.90 kg in the RISPERDAL® 0.5–2.5 mg group, 1.44 kg in the RISPERDAL® 3–6 mg group, and 0.65 kg in the placebo group). A similar trend was observed in the mean change from baseline in body mass index.
When treating pediatric patients with RISPERDAL® for any indication, weight gain should be assessed against that expected with normal growth.
5.6Hyperprolactinemia
As with other drugs that antagonize dopamine D2 receptors, RISPERDAL® elevates prolactin levels and the elevation persists during chronic administration. RISPERDAL® is associated with higher levels of prolactin elevation than other antipsychotic agents.
Hyperprolactinemia may suppress hypothalamic GnRH, resulting in reduced pituitary gonadotropin secretion. This, in turn, may inhibit reproductive function by impairing gonadal steroidogenesis in both female and male patients. Galactorrhea, amenorrhea, gynecomastia, and impotence have been reported in patients receiving prolactin-elevating compounds. Long-standing hyperprolactinemia when associated with hypogonadism may lead to decreased bone density in both female and male subjects.
Tissue culture experiments indicate that approximately one-third of human breast cancers are prolactin dependent in vitro, a factor of potential importance if the prescription of these drugs is contemplated in a patient with previously detected breast cancer. An increase in pituitary gland, mammary gland, and pancreatic islet cell neoplasia (mammary adenocarcinomas, pituitary and pancreatic adenomas) was observed in the risperidone carcinogenicity studies conducted in mice and rats [see Nonclinical Toxicology (13.1)]. Neither clinical studies nor epidemiologic studies conducted to date have shown an association between chronic administration of this class of drugs and tumorigenesis in humans; the available evidence is considered too limited to be conclusive at this time.
5.7Orthostatic Hypotension
RISPERDAL® may induce orthostatic hypotension associated with dizziness, tachycardia, and in some patients, syncope, especially during the initial dose-titration period, probably reflecting its alpha-adrenergic antagonistic properties. Syncope was reported in 0.2% (6/2607) of RISPERDAL®-treated patients in Phase 2 and 3 studies in adults with schizophrenia. The risk of orthostatic hypotension and syncope may be minimized by limiting the initial dose to 2 mg total (either once daily or 1 mg twice daily) in normal adults and 0.5 mg twice daily in the elderly and patients with renal or hepatic impairment [see Dosage and Administration (2.1 , 2.4)]. Monitoring of orthostatic vital signs should be considered in patients for whom this is of concern. A dose reduction should be considered if hypotension occurs. RISPERDAL® should be used with particular caution in patients with known cardiovascular disease (history of myocardial infarction or ischemia, heart failure, or conduction abnormalities), cerebrovascular disease, and conditions which would predispose patients to hypotension, e.g., dehydration and hypovolemia, and in the elderly and patients with renal or hepatic impairment. Monitoring of orthostatic vital signs should be considered if hypotension occurs. Clinically significant hypotension has been observed with concomitant use of RISPERDAL® and antihypertensive medication.
5.8 Falls
Somnolence, postural hypotension, motor and sensory instability have been reported with the use of antipsychotics, including RISPERDAL®, which may lead to falls and, consequently, fractures or other fall-related injuries. For patients, particularly the elderly, with diseases, conditions, or medications that could exacerbate these effects, assess the risk of falls when initiating antipsychotic treatment and recurrently for patients on long-term antipsychotic therapy.
5.9Leukopenia, Neutropenia, and Agranulocytosis
Class Effect: In clinical trial and/or postmarketing experience, events of leukopenia/neutropenia have been reported temporally related to antipsychotic agents, including RISPERDAL®. Agranulocytosis has also been reported.
Possible risk factors for leukopenia/neutropenia include pre-existing low white blood cell count (WBC) and history of drug-induced leukopenia/neutropenia. Patients with a history of a clinically significant low WBC or a drug-induced leukopenia/neutropenia should have their complete blood count (CBC) monitored frequently during the first few months of therapy and discontinuation of RISPERDAL® should be considered at the first sign of a clinically significant decline in WBC in the absence of other causative factors.
Patients with clinically significant neutropenia should be carefully monitored for fever or other symptoms or signs of infection and treated promptly if such symptoms or signs occur. Patients with severe neutropenia (absolute neutrophil count <1000/mm3) should discontinue RISPERDAL® and have their WBC followed until recovery.
5.10Potential for Cognitive and Motor Impairment
Somnolence was a commonly reported adverse reaction associated with RISPERDAL® treatment, especially when ascertained by direct questioning of patients. This adverse reaction is dose-related, and in a study utilizing a checkul to detect adverse events, 41% of the high-dose patients (RISPERDAL® 16 mg/day) reported somnolence compared to 16% of placebo patients. Direct questioning is more sensitive for detecting adverse events than spontaneous reporting, by which 8% of RISPERDAL® 16 mg/day patients and 1% of placebo patients reported somnolence as an adverse reaction. Since RISPERDAL® has the potential to impair judgment, thinking, or motor skills, patients should be cautioned about operating hazardous machinery, including automobiles, until they are reasonably certain that RISPERDAL® therapy does not affect them adversely.
5.11 Seizures
During premarketing testing in adult patients with schizophrenia, seizures occurred in 0.3% (9/2607) of RISPERDAL®-treated patients, two in association with hyponatremia. RISPERDAL® should be used cautiously in patients with a history of seizures.
5.12 Dysphagia
Esophageal dysmotility and aspiration have been associated with antipsychotic drug use. Aspiration pneumonia is a common cause of morbidity and mortality in patients with advanced Alzheimer's dementia. RISPERDAL® and other antipsychotic drugs should be used cautiously in patients at risk for aspiration pneumonia. [see Boxed WarningandWarnings and Precautions (5.1)].
5.13 Priapism
Priapism has been reported during postmarketing surveillance. Severe priapism may require surgical intervention.
5.14 Body Temperature Regulation
Disruption of body temperature regulation has been attributed to antipsychotic agents. Both hyperthermia and hypothermia have been reported in association with oral RISPERDAL® use. Caution is advised when prescribing for patients who will be exposed to temperature extremes.
5.15 Patients with Phenylketonuria
Inform patients that RISPERDAL® M-TAB® Orally Disintegrating Tablets contain phenylalanine. Phenylalanine is a component of aspartame. Each 4 mg RISPERDAL® M-TAB® Orally Disintegrating Tablet contains 0.84 mg phenylalanine; each 3 mg RISPERDAL® M-TAB® Orally Disintegrating Tablet contains 0.63 mg phenylalanine; each 2 mg RISPERDAL® M-TAB® Orally Disintegrating Tablet contains 0.42 mg phenylalanine; each 1 mg RISPERDAL® M-TAB® Orally Disintegrating Tablet contains 0.28 mg phenylalanine; and each 0.5 mg RISPERDAL® M-TAB® Orally Disintegrating Tablet contains 0.14 mg phenylalanine.
6 Adverse Reactions
The following are discussed in more detail in other sections of the labeling:
- Increased mortality in elderly patients with dementia-related psychosis [see Boxed Warning and Warnings and Precautions (5.1)]
- Cerebrovascular adverse events, including stroke, in elderly patients with dementia-related psychosis [see Warnings and Precautions (5.2)]
- Neuroleptic malignant syndrome [see Warnings and Precautions (5.3)]
- Tardive dyskinesia [see Warnings and Precautions (5.4)]
- Metabolic Changes (Hyperglycemia and diabetes mellitus, Dyslipidemia, and Weight Gain) [see Warnings and Precautions (5.5)]
- Hyperprolactinemia [see Warnings and Precautions (5.6)]
- Orthostatic hypotension [see Warnings and Precautions (5.7)]
- Falls [see Warnings and Precautions (5.8)]
- Leukopenia, neutropenia, and agranulocytosis [seeWarnings and Precautions (5.9)]
- Potential for cognitive and motor impairment [seeWarnings and Precautions (5.10)]
- Seizures [see Warnings and Precautions (5.11)]
- Dysphagia [see Warnings and Precautions (5.12)]
- Priapism [see Warnings and Precautions (5.13)]
- Disruption of body temperature regulation [see Warnings and Precautions (5.14)]
- Patients with Phenylketonuria [see Warnings and Precautions (5.15)].
The most common adverse reactions in clinical trials (>5% and twice placebo) were parkinsonism, akathisia, dystonia, tremor, sedation, dizziness, anxiety, blurred vision, nausea, vomiting, upper abdominal pain, stomach discomfort, dyspepsia, diarrhea, salivary hypersecretion, constipation, dry mouth, increased appetite, increased weight, fatigue, rash, nasal congestion, upper respiratory tract infection, nasopharyngitis, and pharyngolaryngeal pain.
The most common adverse reactions that were associated with discontinuation from clinical trials (causing discontinuation in >1% of adults and/or >2% of pediatrics) were nausea, somnolence, sedation, vomiting, dizziness, and akathisia [see Adverse Reactions, Discontinuations Due to Adverse Reactions (6.1)].
The data described in this section are derived from a clinical trial database consisting of 9803 adult and pediatric patients exposed to one or more doses of RISPERDAL® for the treatment of schizophrenia, bipolar mania, autistic disorder, and other psychiatric disorders in pediatrics and elderly patients with dementia. Of these 9803 patients, 2687 were patients who received RISPERDAL® while participating in double-blind, placebo-controlled trials. The conditions and duration of treatment with RISPERDAL® varied greatly and included (in overlapping categories) double-blind, fixed- and flexible-dose, placebo- or active-controlled studies and open-label phases of studies, inpatients and outpatients, and short-term (up to 12 weeks) and longer-term (up to 3 years) exposures. Safety was assessed by collecting adverse events and performing physical examinations, vital signs, body weights, laboratory analyses, and ECGs.
The most common adverse reactions in clinical trials (≥5% and twice placebo) were parkinsonism, akathisia, dystonia, tremor, sedation, dizziness, anxiety, blurred vision, nausea, vomiting, upper abdominal pain, stomach discomfort, dyspepsia, diarrhea, salivary hypersecretion, constipation, dry mouth, increased appetite, increased weight, fatigue, rash, nasal congestion, upper respiratory tract infection, nasopharyngitis, and pharyngolaryngeal pain. (6 )
To report SUSPECTED ADVERSE REACTIONS, contact Janssen Pharmaceuticals, Inc. at 1-800-JANSSEN (1-800-526-7736) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
6.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Commonly-Observed Adverse Reactions in Double-Blind, Placebo-Controlled Clinical Trials – Schizophrenia
Adult Patients with Schizophrenia
Table 8 uls the adverse reactions reported in 2% or more of RISPERDAL®-treated adult patients with schizophrenia in three 4- to 8-week, double-blind, placebo-controlled trials.
Table 8. Adverse Reactions in ≥2% of RISPERDAL®-Treated Adult Patients (and greater than placebo) with Schizophrenia in Double-Blind, Placebo-Controlled Trials Percentage of Patients Reporting Reaction RISPERDAL® System/Organ Class  Adverse Reaction 2–8 mg per day(N=366) >8–16 mg per day(N=198) Placebo(N=225) Cardiac Disorders   Tachycardia 1 3 0 Eye Disorders   Vision blurred 3 1 1 Gastrointestinal Disorders   Nausea 9 4 4   Constipation 8 9 6   Dyspepsia 8 6 5   Dry mouth 4 0 1   Abdominal discomfort 3 1 1   Salivary hypersecretion 2 1 <1   Diarrhea 2 1 1 General Disorders   Fatigue 3 1 0   Chest pain 2 2 1   Asthenia 2 1 <1 Infections and Infestations   Nasopharyngitis 3 4 3   Upper respiratory tract infection 2 3 1   Sinusitis 1 2 1   Urinary tract infection 1 3 0 Investigations   Blood creatine phosphokinase increased 1 2 <1   Heart rate increased <1 2 0 Musculoskeletal and Connective Tissue Disorders   Back pain 4 1 1   Arthralgia 2 3 <1   Pain in extremity 2 1 1 Nervous System Disorders Parkinsonism Parkinsonism includes extrapyramidal disorder, musculoskeletal stiffness, parkinsonism, cogwheel rigidity, akinesia, bradykinesia, hypokinesia, masked facies, muscle rigidity, and Parkinson's disease. Akathisia includes akathisia and restlessness. Dystonia includes dystonia, muscle spasms, muscle contractions involuntary, muscle contracture, oculogyration, tongue paralysis. Tremor includes tremor and parkinsonian rest tremor. 14 17 8   Akathisia 10 10 3   Sedation 10 5 2   Dizziness 7 4 2   Dystonia 3 4 2   Tremor 2 3 1   Dizziness postural 2 0 0 Psychiatric Disorders   Insomnia 32 25 27   Anxiety 16 11 11 Respiratory, Thoracic and Mediastinal Disorders   Nasal congestion 4 6 2   Dyspnea 1 2 0   Epistaxis <1 2 0 Skin and Subcutaneous Tissue Disorders   Rash 1 4 1   Dry skin 1 3 0 Vascular Disorders   Orthostatic hypotension 2 1 0
Pediatric Patients with Schizophrenia
Table 9 uls the adverse reactions reported in 5% or more of RISPERDAL®-treated pediatric patients with schizophrenia in a 6-week double-blind, placebo-controlled trial.
Table 9. Adverse Reactions in ≥5% of RISPERDAL®-Treated Pediatric Patients (and greater than placebo) with Schizophrenia in a Double-Blind Trial Percentage of Patients Reporting Reaction RISPERDAL® System/Organ Class  Adverse Reaction 1–3 mg per day(N=55) 4–6 mg per day(N=51) Placebo(N=54) Gastrointestinal Disorders   Salivary hypersecretion 0 10 2 Nervous System Disorders   Sedation 24 12 4 Parkinsonism Parkinsonism includes extrapyramidal disorder, muscle rigidity, musculoskeletal stiffness, and hypokinesia. Akathisia includes akathisia and restlessness. Dystonia includes dystonia and oculogyration. 16 28 11   Tremor 11 10 6   Akathisia 9 10 4   Dizziness 7 14 2   Dystonia 2 6 0 Psychiatric Disorders   Anxiety 7 6 0
Commonly-Observed Adverse Reactions in Double-Blind, Placebo-Controlled Clinical Trials – Bipolar Mania
Adult Patients with Bipolar Mania
Table 10 uls the adverse reactions reported in 2% or more of RISPERDAL®-treated adult patients with bipolar mania in four 3-week, double-blind, placebo-controlled monotherapy trials.
Table 10. Adverse Reactions in ≥2% of RISPERDAL®-Treated Adult Patients (and greater than placebo) with Bipolar Mania in Double-Blind, Placebo-Controlled Monotherapy Trials Percentage of Patients Reporting Reaction System/Organ Class  Adverse Reaction RISPERDAL® 1–6 mg per day(N=448) Placebo(N=424) Eye Disorders   Vision blurred 2 1 Gastrointestinal Disorders   Nausea 5 2   Diarrhea 3 2   Salivary hypersecretion 3 1   Stomach discomfort 2 <1 General Disorders   Fatigue 2 1 Nervous System Disorders   Parkinsonism Parkinsonism includes extrapyramidal disorder, parkinsonism, musculoskeletal stiffness, hypokinesia, muscle rigidity, muscle tightness, bradykinesia, cogwheel rigidity. Akathisia includes akathisia and restlessness. Tremor includes tremor and parkinsonian rest tremor. Dystonia includes dystonia, muscle spasms, oculogyration, torticollis. 25 9   Sedation 11 4   Akathisia 9 3   Tremor 6 3   Dizziness 6 5   Dystonia 5 1   Lethargy 2 1
Table 11 uls the adverse reactions reported in 2% or more of RISPERDAL®-treated adult patients with bipolar mania in two 3-week, double-blind, placebo-controlled adjuvant therapy trials.
Table 11. Adverse Reactions in ≥2% of RISPERDAL®-Treated Adult Patients (and greater than placebo) with Bipolar Mania in Double-Blind, Placebo-Controlled Adjunctive Therapy Trials Percentage of Patients Reporting Reaction System/Organ Class RISPERDAL® + Mood Stabilizer Placebo + Mood Stabilizer   Adverse Reaction (N=127) (N=126) Cardiac Disorders   Palpitations 2 0 Gastrointestinal Disorders   Dyspepsia 9 8   Nausea 6 4   Diarrhea 6 4   Salivary hypersecretion 2 0 General Disorders   Chest pain 2 1 Infections and Infestations   Urinary tract infection 2 1 Nervous System Disorders   Parkinsonism Parkinsonism includes extrapyramidal disorder, hypokinesia and bradykinesia. Akathisia includes hyperkinesia and akathisia. 14 4   Sedation 9 4   Akathisia 8 0   Dizziness 7 2   Tremor 6 2   Lethargy 2 1 Psychiatric Disorders   Anxiety 3 2 Respiratory, Thoracic and Mediastinal Disorders   Pharyngolaryngeal pain 5 2   Cough 2 0
Pediatric Patients with Bipolar Mania
Table 12 uls the adverse reactions reported in 5% or more of RISPERDAL®-treated pediatric patients with bipolar mania in a 3-week double-blind, placebo-controlled trial.
Table 12. Adverse Reactions in ≥5% of RISPERDAL®-Treated Pediatric Patients (and greater than placebo) with Bipolar Mania in Double-Blind, Placebo-Controlled Trials Percentage of Patients Reporting Reaction RISPERDAL® System/Organ Class  Adverse Reaction 0.5–2.5 mg per day(N=50) 3–6 mg per day(N=61) Placebo(N=58) Eye Disorders   Vision blurred 4 7 0 Gastrointestinal Disorders   Abdominal pain upper 16 13 5   Nausea 16 13 7   Vomiting 10 10 5   Diarrhea 8 7 2   Dyspepsia 10 3 2   Stomach discomfort 6 0 2 General Disorders   Fatigue 18 30 3 Metabolism and Nutrition Disorders   Increased appetite 4 7 2 Nervous System Disorders   Sedation 42 56 19   Dizziness 16 13 5 Parkinsonism Parkinsonism includes musculoskeletal stiffness, extrapyramidal disorder, bradykinesia, and nuchal rigidity. Dystonia includes dystonia, laryngospasm, and muscle spasms. Akathisia includes restlessness and akathisia. 6 12 3   Dystonia 6 5 0   Akathisia 0 8 2 Psychiatric Disorders   Anxiety 0 8 3 Respiratory, Thoracic and Mediastinal Disorders   Pharyngolaryngeal pain 10 3 5 Skin and Subcutaneous Tissue Disorders   Rash 0 7 2
Commonly-Observed Adverse Reactions in Double-Blind, Placebo-Controlled Clinical Trials - Autistic Disorder
Table 13 uls the adverse reactions reported in 5% or more of RISPERDAL®-treated pediatric patients treated for irritability associated with autistic disorder in two 8-week, double-blind, placebo-controlled trials and one 6-week double-blind, placebo-controlled study.
Table 13. Adverse Reactions in ≥5% of RISPERDAL®-Treated Pediatric Patients (and greater than placebo) Treated for Irritability Associated with Autistic Disorder in Double-Blind, Placebo-Controlled Trials Percentage of Patients Reporting Reaction System/Organ Class RISPERDAL® 0.5–4.0 mg/day Placebo   Adverse Reaction (N=107) (N=115) Gastrointestinal Disorders   Vomiting 20 17   Constipation 17 6   Dry mouth 10 4   Nausea 8 5   Salivary hypersecretion 7 1 General Disorders and Administration Site Conditions   Fatigue 31 9   Pyrexia 16 13   Thirst 7 4 Infections and Infestations   Nasopharyngitis 19 9   Rhinitis 9 7   Upper respiratory tract infection 8 3 Investigations   Weight increased 8 2 Metabolism and Nutrition Disorders   Increased appetite 44 15 Nervous System Disorders   Sedation 63 15   Drooling 12 4   Headache 12 10   Tremor 8 1   Dizziness 8 2   Parkinsonism Parkinsonism includes musculoskeletal stiffness, extrapyramidal disorder, muscle rigidity, cogwheel rigidity, and muscle tightness. 8 1 Renal and Urinary Disorders   Enuresis 16 10 Respiratory, Thoracic and Mediastinal Disorders   Cough 17 12   Rhinorrhea 12 10   Nasal congestion 10 4 Skin and Subcutaneous Tissue Disorders   Rash 8 5
Other Adverse Reactions Observed During the Clinical Trial Evaluation of Risperidone
The following additional adverse reactions occurred across all placebo-controlled, active-controlled, and open-label studies of RISPERDAL® in adults and pediatric patients.
Blood and Lymphatic System Disorders: anemia, granulocytopenia, neutropenia
Cardiac Disorders: sinus bradycardia, sinus tachycardia, atrioventricular block first degree, bundle branch block left, bundle branch block right, atrioventricular block
Ear and Labyrinth Disorders: ear pain, tinnitus
Endocrine Disorders: hyperprolactinemia
Eye Disorders: ocular hyperemia, eye discharge, conjunctivitis, eye rolling, eyelid edema, eye swelling, eyelid margin crusting, dry eye, lacrimation increased, photophobia, glaucoma, visual acuity reduced
Gastrointestinal Disorders: dysphagia, fecaloma, fecal incontinence, gastritis, lip swelling, cheilitis, aptyalism
General Disorders: edema peripheral, thirst, gait disturbance, influenza-like illness, pitting edema, edema, chills, sluggishness, malaise, chest discomfort, face edema, discomfort, generalized edema, drug withdrawal syndrome, peripheral coldness, feeling abnormal
Immune System Disorders: drug hypersensitivity
Infections and Infestations: pneumonia, influenza, ear infection, viral infection, pharyngitis, tonsillitis, bronchitis, eye infection, localized infection, cystitis, cellulitis, otitis media, onychomycosis, acarodermatitis, bronchopneumonia, respiratory tract infection, tracheobronchitis, otitis media chronic
Investigations: body temperature increased, blood prolactin increased, alanine aminotransferase increased, electrocardiogram abnormal, eosinophil count increased, white blood cell count decreased, blood glucose increased, hemoglobin decreased, hematocrit decreased, body temperature decreased, blood pressure decreased, transaminases increased
Metabolism and Nutrition Disorders: decreased appetite, polydipsia, anorexia
Musculoskeletal and Connective Tissue Disorders: joint stiffness, joint swelling, musculoskeletal chest pain, posture abnormal, myalgia, neck pain, muscular weakness, rhabdomyolysis
Nervous System Disorders: balance disorder, disturbance in attention, dysarthria, unresponsive to stimuli, depressed level of consciousness, movement disorder, transient ischemic attack, coordination abnormal, cerebrovascular accident, speech disorder, syncope, loss of consciousness, hypoesthesia, tardive dyskinesia, dyskinesia, cerebral ischemia, cerebrovascular disorder, neuroleptic malignant syndrome, diabetic coma, head titubation
Psychiatric Disorders: agitation, blunted affect, confusional state, middle insomnia, nervousness, sleep disorder, ullessness, libido decreased, and anorgasmia
Renal and Urinary Disorders: enuresis, dysuria, pollakiuria, urinary incontinence
Reproductive System and Breast Disorders: menstruation irregular, amenorrhea, gynecomastia, galactorrhea, vaginal discharge, menstrual disorder, erectile dysfunction, retrograde ejaculation, ejaculation disorder, sexual dysfunction, breast enlargement
Respiratory, Thoracic, and Mediastinal Disorders: wheezing, pneumonia aspiration, sinus congestion, dysphonia, productive cough, pulmonary congestion, respiratory tract congestion, rales, respiratory disorder, hyperventilation, nasal edema
Skin and Subcutaneous Tissue Disorders: erythema, skin discoloration, skin lesion, pruritus, skin disorder, rash erythematous, rash papular, rash generalized, rash maculopapular, acne, hyperkeratosis, seborrheic dermatitis
Vascular Disorders: hypotension, flushing
Additional Adverse Reactions Reported with RISPERDAL CONSTA®
The following is a ul of additional adverse reactions that have been reported during the premarketing evaluation of RISPERDAL CONSTA®, regardless of frequency of occurrence:
Cardiac Disorders: bradycardia
Ear and Labyrinth Disorders: vertigo
Eye Disorders: blepharospasm
Gastrointestinal Disorders: toothache, tongue spasm
General Disorders and Administration Site Conditions: pain
Infections and Infestations: lower respiratory tract infection, infection, gastroenteritis, subcutaneous abscess
Injury and Poisoning: fall
Investigations: weight decreased, gamma-glutamyltransferase increased, hepatic enzyme increased
Musculoskeletal, Connective Tissue, and Bone Disorders: buttock pain
Nervous System Disorders: convulsion, paresthesia
Psychiatric Disorders: depression
Skin and Subcutaneous Tissue Disorders: eczema
Vascular Disorders: hypertension
Discontinuations Due to Adverse Reactions
Schizophrenia - Adults
Approximately 7% (39/564) of RISPERDAL®-treated patients in double-blind, placebo-controlled trials discontinued treatment due to an adverse reaction, compared with 4% (10/225) who were receiving placebo. The adverse reactions associated with discontinuation in 2 or more RISPERDAL®-treated patients were:
Table 14. Adverse Reactions Associated With Discontinuation in 2 or More RISPERDAL®-Treated Adult Patients in Schizophrenia Trials RISPERDAL® Adverse Reaction 2–8 mg/day(N=366) >8–16 mg/day(N=198) Placebo(N=225) Dizziness 1.4% 1.0% 0% Nausea 1.4% 0% 0% Vomiting 0.8% 0% 0% Parkinsonism 0.8% 0% 0% Somnolence 0.8% 0% 0% Dystonia 0.5% 0% 0% Agitation 0.5% 0% 0% Abdominal pain 0.5% 0% 0% Orthostatic hypotension 0.3% 0.5% 0% Akathisia 0.3% 2.0% 0%
Discontinuation for extrapyramidal symptoms (including Parkinsonism, akathisia, dystonia, and tardive dyskinesia) was 1% in placebo-treated patients, and 3.4% in active control-treated patients in a double-blind, placebo- and active-controlled trial.
Schizophrenia - Pediatrics
Approximately 7% (7/106), of RISPERDAL®-treated patients discontinued treatment due to an adverse reaction in a double-blind, placebo-controlled trial, compared with 4% (2/54) placebo-treated patients. The adverse reactions associated with discontinuation for at least one RISPERDAL®-treated patient were dizziness (2%), somnolence (1%), sedation (1%), lethargy (1%), anxiety (1%), balance disorder (1%), hypotension (1%), and palpitation (1%).
Bipolar Mania - Adults
In double-blind, placebo-controlled trials with RISPERDAL® as monotherapy, approximately 6% (25/448) of RISPERDAL®-treated patients discontinued treatment due to an adverse event, compared with approximately 5% (19/424) of placebo-treated patients. The adverse reactions associated with discontinuation in RISPERDAL®-treated patients were:
Table 15. Adverse Reactions Associated With Discontinuation in 2 or More RISPERDAL®-Treated Adult Patients in Bipolar Mania Clinical Trials Adverse Reaction RISPERDAL® 1–6 mg/day(N=448) Placebo(N=424) Parkinsonism 0.4% 0% Lethargy 0.2% 0% Dizziness 0.2% 0% Alanine aminotransferase increased 0.2% 0.2% Aspartate aminotransferase increased 0.2% 0.2%
Bipolar Mania - Pediatrics
In a double-blind, placebo-controlled trial 12% (13/111) of RISPERDAL®-treated patients discontinued due to an adverse reaction, compared with 7% (4/58) of placebo-treated patients. The adverse reactions associated with discontinuation in more than one RISPERDAL®-treated pediatric patient were nausea (3%), somnolence (2%), sedation (2%), and vomiting (2%).
Autistic Disorder - Pediatrics
In the two 8-week, placebo-controlled trials in pediatric patients treated for irritability associated with autistic disorder (n=156), one RISPERDAL®-treated patient discontinued due to an adverse reaction (Parkinsonism), and one placebo-treated patient discontinued due to an adverse event.
Dose Dependency of Adverse Reactions in Clinical Trials
Extrapyramidal Symptoms
Data from two fixed-dose trials in adults with schizophrenia provided evidence of dose-relatedness for extrapyramidal symptoms associated with RISPERDAL® treatment.
Two methods were used to measure extrapyramidal symptoms (EPS) in an 8-week trial comparing 4 fixed doses of RISPERDAL® (2, 6, 10, and 16 mg/day), including (1) a Parkinsonism score (mean change from baseline) from the Extrapyramidal Symptom Rating Scale, and (2) incidence of spontaneous complaints of EPS:
Table 16. Dose Groups Placebo RISPERDAL® 2 mg RISPERDAL® 6 mg RISPERDAL® 10 mg RISPERDAL® 16 mg Parkinsonism 1.2 0.9 1.8 2.4 2.6 EPS Incidence 13% 17% 21% 21% 35%
Similar methods were used to measure extrapyramidal symptoms (EPS) in an 8-week trial comparing 5 fixed doses of RISPERDAL® (1, 4, 8, 12, and 16 mg/day):
Table 17. Dose Groups RISPERDAL® 1 mg RISPERDAL® 4 mg RISPERDAL® 8 mg RISPERDAL® 12 mg RISPERDAL® 16 mg Parkinsonism 0.6 1.7 2.4 2.9 4.1 EPS Incidence 7% 12% 17% 18% 20%
Dystonia
Other Adverse Reactions
Adverse event data elicited by a checkul for side effects from a large study comparing 5 fixed doses of RISPERDAL® (1, 4, 8, 12, and 16 mg/day) were explored for dose-relatedness of adverse events. A Cochran-Armitage Test for trend in these data revealed a positive trend (p<0.05) for the following adverse reactions: somnolence, vision abnormal, dizziness, palpitations, weight increase, erectile dysfunction, ejaculation disorder, sexual function abnormal, fatigue, and skin discoloration.
Changes in Body Weight
Weight gain was observed in short-term, controlled trials and longer-term uncontrolled studies in adult and pediatric patients [seeWarnings and Precautions (5.5),Adverse Reactions (6), andUse in Specific Populations (8.4)].
Changes in ECG Parameters
Between-group comparisons for pooled placebo-controlled trials in adults revealed no statistically significant differences between risperidone and placebo in mean changes from baseline in ECG parameters, including QT, QTc, and PR intervals, and heart rate. When all RISPERDAL® doses were pooled from randomized controlled trials in several indications, there was a mean increase in heart rate of 1 beat per minute compared to no change for placebo patients. In short-term schizophrenia trials, higher doses of risperidone (8–16 mg/day) were associated with a higher mean increase in heart rate compared to placebo (4–6 beats per minute). In pooled placebo-controlled acute mania trials in adults, there were small decreases in mean heart rate, similar among all treatment groups.
In the two placebo-controlled trials in children and adolescents with autistic disorder (aged 5 – 16 years) mean changes in heart rate were an increase of 8.4 beats per minute in the RISPERDAL® groups and 6.5 beats per minute in the placebo group. There were no other notable ECG changes.
In a placebo-controlled acute mania trial in children and adolescents (aged 10 – 17 years), there were no significant changes in ECG parameters, other than the effect of RISPERDAL® to transiently increase pulse rate (< 6 beats per minute). In two controlled schizophrenia trials in adolescents (aged 13 – 17 years), there were no clinically meaningful changes in ECG parameters including corrected QT intervals between treatment groups or within treatment groups over time.
6.2Postmarketing Experience
The following adverse reactions have been identified during postapproval use of risperidone. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These adverse reactions include: alopecia, anaphylactic reaction, angioedema, atrial fibrillation, cardiopulmonary arrest, catatonia, diabetic ketoacidosis in patients with impaired glucose metabolism, dysgeusia, hypoglycemia, hypothermia, ileus, inappropriate antidiuretic hormone secretion, intestinal obstruction, jaundice, mania, pancreatitis, pituitary adenoma, precocious puberty, pulmonary embolism, QT prolongation, sleep apnea syndrome, somnambulism, Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN), sudden death, thrombocytopenia, thrombotic thrombocytopenic purpura, urinary retention, and water intoxication.
Postmarketing cases of extrapyramidal symptoms (dystonia and dyskinesia) have been reported in patients concomitantly taking methylphenidate and risperidone when there was an increase or decrease in dosage, initiation, or discontinuation of either or both medications.
7 Drug Interactions
- Carbamazepine and other enzyme inducers decrease plasma concentrations of risperidone. Increase the RISPERDAL® dose up to double the patient's usual dose. Titrate slowly. (
7.1 )- Fluoxetine, paroxetine, and other CYP 2D6 enzyme inhibitors increase plasma concentrations of risperidone. Reduce the initial dose. Do not exceed a final dose of 8 mg per day of RISPERDAL®. (
7.1 )7.1Pharmacokinetic-related Interactions
The dose of RISPERDAL® should be adjusted when used in combination with CYP2D6 enzyme inhibitors (e.g., fluoxetine, and paroxetine) and enzyme inducers (e.g., carbamazepine) [seeTable 18andDosage and Administration (2.5)]. Dose adjustment is not recommended for RISPERDAL® when co-administered with ranitidine, cimetidine, amitriptyline, or erythromycin [seeTable 18].
Table 18. Summary of Effect of Coadministered Drugs on Exposure to Active Moiety (Risperidone + 9-Hydroxy-Risperidone) in Healthy Subjects or Patients with Schizophrenia Coadministered Drug Dosing Schedule Effect on Active Moiety (Risperidone + 9-Hydroxy-Risperidone (Ratio Change relative to reference )Risperidone Dose Recommendation Coadministered Drug Risperidone AUC Cmax Enzyme (CYP2D6) Inhibitors Fluoxetine 20 mg/day 2 or 3 mg twice daily 1.4 1.5 Re-evaluate dosing. Do not exceed 8 mg/day Paroxetine 10 mg/day 4 mg/day 1.3 - Re-evaluate dosing. Do not exceed 8 mg/day 20 mg/day 4 mg/day 1.6 - 40 mg/day 4 mg/day 1.8 - Enzyme (CYP3A/PgP inducers) Inducers Carbamazepine 573 ± 168 mg/day 3 mg twice daily 0.51 0.55 Titrate dose upwards. Do not exceed twice the patient's usual dose Enzyme (CYP3A) Inhibitors Ranitidine 150 mg twice daily 1 mg single dose 1.2 1.4 Dose adjustment not needed Cimetidine 400 mg twice daily 1 mg single dose 1.1 1.3 Dose adjustment not needed Erythromycin 500 mg four times daily 1 mg single dose 1.1 0.94 Dose adjustment not needed Other Drugs Amitriptyline 50 mg twice daily 3 mg twice daily 1.2 1.1 Dose adjustment not needed
Effect of Risperidone on Other Drugs
Lithium
Repeated oral doses of RISPERDAL® (3 mg twice daily) did not affect the exposure (AUC) or peak plasma concentrations (Cmax) of lithium (n=13). Dose adjustment for lithium is not recommended.
Valproate
Repeated oral doses of RISPERDAL® (4 mg once daily) did not affect the pre-dose or average plasma concentrations and exposure (AUC) of valproate (1000 mg/day in three divided doses) compared to placebo (n=21). However, there was a 20% increase in valproate peak plasma concentration (Cmax) after concomitant administration of RISPERDAL®. Dose adjustment for valproate is not recommended.
Digoxin
RISPERDAL® (0.25 mg twice daily) did not show a clinically relevant effect on the pharmacokinetics of digoxin. Dose adjustment for digoxin is not recommended.
7.2Pharmacodynamic-related Interactions
Centrally Acting Drugs and Alcohol
Given the primary CNS effects of risperidone, caution should be used when RISPERDAL® is taken in combination with other centrally acting drugs and alcohol.
Drugs with Hypotensive Effects
Because of its potential for inducing hypotension, RISPERDAL® may enhance the hypotensive effects of other therapeutic agents with this potential.
Levodopa and Dopamine Agonists
RISPERDAL® may antagonize the effects of levodopa and dopamine agonists.
Methylphenidate
Concomitant use with methylphenidate, when there is change in dosage of either medication, may increase the risk of extrapyramidal symptoms (EPS). Monitor for symptoms of EPS with concomitant use of RISPERDAL® and methylphenidate [see Adverse Reactions (6.2)].
Clozapine
Chronic administration of clozapine with RISPERDAL® may decrease the clearance of risperidone.
8 Use In Specific Populations
- Pregnancy: May cause extrapyramidal and/or withdrawal symptoms in neonates with third trimester exposure. (
8.1 )8.1Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to atypical antipsychotics, including RISPERDAL®, during pregnancy. Healthcare providers are encouraged to register patients by contacting the National Pregnancy Registry for Atypical Antipsychotics at 1-866-961-2388 or online at http://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/.
Risk Summary
Neonates exposed to antipsychotic drugs during the third trimester of pregnancy are at risk for extrapyramidal and/or withdrawal symptoms following delivery (see Clinical Considerations). Overall, available data from published epidemiologic studies of pregnant women exposed to risperidone have not established a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes (see Data). There are risks to the mother associated with untreated schizophrenia or bipolar I disorder and with exposure to antipsychotics, including RISPERDAL®, during pregnancy (see Clinical Considerations).
Oral administration of risperidone to pregnant mice caused cleft palate at doses 3 to 4 times the maximum recommended human dose (MRHD) with maternal toxicity observed at 4-times MRHD based on mg/m2 body surface area. Risperidone was not teratogenic in rats or rabbits at doses up to 6-times the MRHD based on mg/m2 body surface area. Increased stillbirths and decreased birth weight occurred after oral risperidone administration to pregnant rats at 1.5-times the MRHD based on mg/m2 body surface area. Learning was impaired in offspring of rats when the dams were dosed at 0.6-times the MRHD and offspring mortality increased at doses 0.1 to 3 times the MRHD based on mg/m2 body surface area.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2–4% and 15–20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
There is a risk to the mother from untreated schizophrenia or bipolar I disorder, including increased risk of relapse, hospitalization, and suicide. Schizophrenia and bipolar I disorder are associated with increased adverse perinatal outcomes, including preterm birth. It is not known if this is a direct result of the illness or other comorbid factors.
Fetal/Neonatal Adverse Reactions
Extrapyramidal and/or withdrawal symptoms, including agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress, and feeding disorder have been reported in neonates who were exposed to antipsychotic drugs, including RISPERDAL®, during the third trimester of pregnancy. These symptoms have varied in severity. Monitor neonates for extrapyramidal and/or withdrawal symptoms and manage symptoms appropriately. Some neonates recovered within hours or days without specific treatment; others required prolonged hospitalization.
Data
Human Data
Published data from observational studies, birth registries, and case reports on the use of atypical antipsychotics during pregnancy do not report a clear association with antipsychotics and major birth defects. A prospective observational study including 6 women treated with risperidone demonstrated placental passage of risperidone. A retrospective cohort study from a Medicaid database of 9258 women exposed to antipsychotics during pregnancy did not indicate an overall increased risk for major birth defects. There was a small increase in the risk of major birth defects (RR=1.26, 95% CI 1.02–1.56) and of cardiac malformations (RR=1.26, 95% CI 0.88–1.81) in a subgroup of 1566 women exposed to risperidone during the first trimester of pregnancy; however, there is no mechanism of action to explain the difference in malformation rates.
Animal Data
Oral administration of risperidone to pregnant mice during organogenesis caused cleft palate at 10 mg/kg/day which is 3 times the MRHD of 16 mg/day based on mg/m2 body surface area: maternal toxicity occurred at 4 times the MRHD. Risperidone was not teratogenic when administered orally to rats at 0.6 to 10 mg/kg/day and rabbits at 0.3 to 5 mg/kg/day, which are up to 6 times the MRHD of 16 mg/day risperidone based on mg/m2 body surface area. Learning was impaired in offspring of rats dosed orally throughout pregnancy at 1 mg/kg/day which is 0.6 times the MRHD and neuronal cell death increased in fetal brains of offspring of rats dosed during pregnancy at 1 and 2 mg/kg/day which are 0.6 and 1.2 times the MRHD based on mg/m2 body surface area; postnatal development and growth of the offspring were also delayed.
Rat offspring mortality increased during the first 4 days of lactation when pregnant rats were dosed throughout gestation at 0.16 to 5 mg/kg/day which are 0.1 to 3 times the MRHD of 16 mg/day based on mg/m2 body surface area. It is not known whether these deaths were due to a direct effect on the fetuses or pups or to effects on the dams; a no-effect dose could not be determined. The rate of stillbirths was increased at 2.5 mg/kg or 1.5 times the MRHD based on mg/m2 body surface area.
In a rat cross-fostering study the number of live offspring was decreased, the number of stillbirths increased, and the birth weight was decreased in offspring of drug-treated pregnant rats. In addition, the number of deaths increased by Day 1 among offspring of drug-treated pregnant rats, regardless of whether or not the offspring were cross-fostered. Risperidone also appeared to impair maternal behavior in that offspring body weight gain and survival (from Day 1 to 4 of lactation) were reduced in offspring born to control but reared by drug-treated dams. All of these effects occurred at 5 mg/kg which is 3 times the MRHD based on mg/m2 and the only dose tested in the study.
8.2 Lactation
Risk Summary
Limited data from published literature reports the presence of risperidone and its metabolite, 9-hydroxyrisperidone, in human breast milk at relative infant dose ranging between 2.3% and 4.7% of the maternal weight-adjusted dosage. There are reports of sedation, failure to thrive, jitteriness, and extrapyramidal symptoms (tremors and abnormal muscle movements) in breastfed infants exposed to risperidone (see Clinical Considerations). There is no information on the effects of risperidone on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for RISPERDAL® and any potential adverse effects on the breastfed child from RISPERDAL® or from the mother's underlying condition.
Clinical Considerations
Infants exposed to RISPERDAL® through breastmilk should be monitored for excess sedation, failure to thrive, jitteriness, and extrapyramidal symptoms (tremors and abnormal muscle movements).
8.3 Females and Males of Reproductive Potential
Infertility
Females
Based on the pharmacologic action of risperidone (D2 receptor antagonism), treatment with RISPERDAL® may result in an increase in serum prolactin levels, which may lead to a reversible reduction in fertility in females of reproductive potential [see Warnings and Precautions (5.6)].
8.4 Pediatric Use
Approved Pediatric Indications
Schizophrenia
The efficacy and safety of RISPERDAL® in the treatment of schizophrenia were demonstrated in 417 adolescents, aged 13 to 17 years, in two short-term (6 and 8 weeks, respectively) double-blind controlled trials [see Indications and Usage (1.1) , Adverse Reactions (6.1) , and Clinical Studies (14.1)]. Additional safety and efficacy information was also assessed in one long-term (6-month) open-label extension study in 284 of these adolescent patients with schizophrenia.
Safety and effectiveness of RISPERDAL® in children less than 13 years of age with schizophrenia have not been established.
Bipolar I Disorder
The efficacy and safety of RISPERDAL® in the short-term treatment of acute manic or mixed episodes associated with Bipolar I Disorder in 169 children and adolescent patients, aged 10 to 17 years, were demonstrated in one double-blind, placebo-controlled, 3-week trial [see Indications and Usage (1.2) , Adverse Reactions (6.1) , and Clinical Studies (14.2)].
Safety and effectiveness of RISPERDAL® in children less than 10 years of age with bipolar disorder have not been established.
Autistic Disorder
The efficacy and safety of RISPERDAL® in the treatment of irritability associated with autistic disorder were established in two 8-week, double-blind, placebo-controlled trials in 156 children and adolescent patients, aged 5 to 16 years [see Indications and Usage (1.3) , Adverse Reactions (6.1) and Clinical Studies (14.4) ]. Additional safety information was also assessed in a long-term study in patients with autistic disorder, or in short- and long-term studies in more than 1200 pediatric patients with psychiatric disorders other than autistic disorder, schizophrenia, or bipolar mania who were of similar age and weight, and who received similar dosages of RISPERDAL® as patients treated for irritability associated with autistic disorder.
A third study was a 6-week, multicenter, randomized, double-blind, placebo-controlled, fixed-dose study to evaluate the efficacy and safety of a lower than recommended dose of risperidone in subjects 5 to 17 years of age with autistic disorder and associated irritability, and related behavioral symptoms. There were two weight-based, fixed doses of risperidone (high-dose and low-dose). The high dose was 1.25 mg per day for patients weighing 20 to < 45 kg, and it was 1.75 mg per day for patients weighing ≥ 45 kg. The low dose was 0.125 mg per day for patients for patients weighing 20 to < 45 kg, and it was 0.175 mg per day for patients weighing ≥ 45 kg. The study demonstrated the efficacy of high-dose risperidone, but it did not demonstrate efficacy for low-dose risperidone.
Adverse Reactions in Pediatric Patients
Tardive Dyskinesia
In clinical trials in 1885 children and adolescents treated with RISPERDAL®, 2 (0.1%) patients were reported to have tardive dyskinesia, which resolved on discontinuation of RISPERDAL® treatment [see also Warnings and Precautions (5.4)].
Weight Gain
Weight gain has been observed in children and adolescents during treatment with RISPERDAL®. Clinical monitoring of weight is recommended during treatment.
Data derive from short-term placebo-controlled trials and longer-term uncontrolled studies in pediatric patients (ages 5 to 17 years) with schizophrenia, bipolar disorder, autistic disorder, or other psychiatric disorders. In the short-term trials (3 to 8 weeks), the mean weight gain for RISPERDAL®-treated patients was 2 kg, compared to 0.6 kg for placebo-treated patients. In these trials, approximately 33% of the RISPERDAL® group had weight gain ≥7%, compared to 7% in the placebo group. In longer-term, uncontrolled, open-label pediatric studies, the mean weight gain was 5.5 kg at Week 24 and 8 kg at Week 48 [seeWarnings and Precautions (5.5)andAdverse Reactions (6.1)].
Somnolence
Somnolence was frequently observed in placebo-controlled clinical trials of pediatric patients with autistic disorder. Most cases were mild or moderate in severity. These events were most often of early onset with peak incidence occurring during the first two weeks of treatment, and transient with a median duration of 16 days. Somnolence was the most commonly observed adverse reaction in the clinical trial of bipolar disorder in children and adolescents, as well as in the schizophrenia trials in adolescents. As was seen in the autistic disorder trials, these adverse reactions were most often of early onset and transient in duration [seeAdverse Reactions (6.1and6.2)]. Patients experiencing persistent somnolence may benefit from a change in dosing regimen [see Dosage and Administration (2.1 , 2.2 , and2.3)].
Hyperprolactinemia
RISPERDAL® has been shown to elevate prolactin levels in children and adolescents as well as in adults [see Warnings and Precautions (5.6)]. In double-blind, placebo-controlled studies of up to 8 weeks duration in children and adolescents (aged 5 to 17 years) with autistic disorder or psychiatric disorders other than autistic disorder, schizophrenia, or bipolar mania, 49% of patients who received RISPERDAL® had elevated prolactin levels compared to 2% of patients who received placebo. Similarly, in placebo-controlled trials in children and adolescents (aged 10 to 17 years) with bipolar disorder, or adolescents (aged 13 to 17 years) with schizophrenia, 82–87% of patients who received RISPERDAL® had elevated levels of prolactin compared to 3–7% of patients on placebo. Increases were dose-dependent and generally greater in females than in males across indications.
In clinical trials in 1885 children and adolescents, galactorrhea was reported in 0.8% of RISPERDAL®-treated patients and gynecomastia was reported in 2.3% of RISPERDAL®-treated patients.
Growth and Sexual Maturation
The long-term effects of RISPERDAL® on growth and sexual maturation have not been fully evaluated in children and adolescents.
Juvenile Animal Studies
Juvenile dogs were treated with oral risperidone from weeks 10 to 50 of age (equivalent to the period of childhood through adolescence in humans), at doses of 0.31, 1.25, or 5 mg/kg/day, which are 1.2, 3.4, and 13.5 times the MRHD of 6 mg/day for children, based on mg/m2 body surface area. Bone length and density were decreased with a no-effect dose of 0.31 mg/kg/day; this dose produced plasma AUC of risperidone plus its active metabolite paliperidone (9-hydroxy-risperidone) that were similar to those in children and adolescents receiving the MRHD of 6 mg/day. In addition, sexual maturation was delayed at all doses in both males and females. The above effects showed little or no reversibility in females after a 12 week drug-free recovery period.
Juvenile rats, treated with oral risperidone from days 12 to 50 of age (equivalent to the period of infancy through adolescence in humans) showed impaired learning and memory performance (reversible only in females), with a no-effect dose of 0.63 mg/kg/day which is 0.5 times the MRHD of 6 mg/day for children, based on mg/m2 body surface area. This dose produced plasma AUC of risperidone plus paliperidone about half the exposure observed in humans at the MRHD. No other consistent effects on neurobehavioral or reproductive development were seen up to the highest tested dose of 1.25 mg/kg/day which is 1 time the MRHD and produced plasma AUC of risperidone plus paliperidone that were about two thirds of those observed in humans at the MRHD of 6 mg/day for children.
8.5Geriatric Use
Clinical studies of RISPERDAL® in the treatment of schizophrenia did not include sufficient numbers of patients aged 65 and over to determine whether or not they respond differently than younger patients. Other reported clinical experience has not identified differences in responses between elderly and younger patients. In general, a lower starting dose is recommended for an elderly patient, reflecting a decreased pharmacokinetic clearance in the elderly, as well as a greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy [see Clinical Pharmacology (12.3) and Dosage and Administration (2.4, 2.5)]. While elderly patients exhibit a greater tendency to orthostatic hypotension, its risk in the elderly may be minimized by limiting the initial dose to 0.5 mg twice daily followed by careful titration [see Warnings and Precautions (5.7)]. Monitoring of orthostatic vital signs should be considered in patients for whom this is of concern.
This drug is substantially excreted by the kidneys, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function [see Dosage and Administration (2.4)].
8.6Renal Impairment
In patients with moderate to severe (Clcr 59 to 15 mL/min) renal disease, clearance of the sum of risperidone and its active metabolite decreased by 60%, compared to young healthy subjects. RISPERDAL® doses should be reduced in patients with renal disease [see Dosage and Administration (2.4)].
8.7Hepatic Impairment
While the pharmacokinetics of risperidone in subjects with liver disease were comparable to those in young healthy subjects, the mean free fraction of risperidone in plasma was increased by about 35% because of the diminished concentration of both albumin and α1-acid glycoprotein. RISPERDAL® doses should be reduced in patients with liver disease [see Dosage and Administration (2.4)].
8.8Patients with Parkinson's Disease or Lewy Body Dementia
Patients with Parkinson's Disease or Dementia with Lewy Bodies can experience increased sensitivity to RISPERDAL®. Manifestations can include confusion, obtundation, postural instability with frequent falls, extrapyramidal symptoms, and clinical features consistent with neuroleptic malignant syndrome.
9 Drug Abuse And Dependence
9.1Controlled Substance
RISPERDAL® (risperidone) is not a controlled substance.
9.2Abuse
RISPERDAL® has not been systematically studied in animals or humans for its potential for abuse. While the clinical trials did not reveal any tendency for any drug-seeking behavior, these observations were not systematic and it is not possible to predict on the basis of this limited experience the extent to which a CNS-active drug will be misused, diverted, and/or abused once marketed. Consequently, patients should be evaluated carefully for a history of drug abuse, and such patients should be observed closely for signs of RISPERDAL® misuse or abuse (e.g., development of tolerance, increases in dose, drug-seeking behavior).
9.3Dependence
RISPERDAL® has not been systematically studied in animals or humans for its potential for tolerance or physical dependence.
10 Overdosage
10.1Human Experience
Premarketing experience included eight reports of acute RISPERDAL® overdosage with estimated doses ranging from 20 to 300 mg and no fatalities. In general, reported signs and symptoms were those resulting from an exaggeration of the drug's known pharmacological effects, i.e., drowsiness and sedation, tachycardia and hypotension, and extrapyramidal symptoms. One case, involving an estimated overdose of 240 mg, was associated with hyponatremia, hypokalemia, prolonged QT, and widened QRS. Another case, involving an estimated overdose of 36 mg, was associated with a seizure.
Postmarketing experience includes reports of acute RISPERDAL® overdosage, with estimated doses of up to 360 mg. In general, the most frequently reported signs and symptoms are those resulting from an exaggeration of the drug's known pharmacological effects, i.e., drowsiness, sedation, tachycardia, hypotension, and extrapyramidal symptoms. Other adverse reactions reported since market introduction related to RISPERDAL® overdose include prolonged QT interval and convulsions. Torsade de pointes has been reported in association with combined overdose of RISPERDAL® and paroxetine.
10.2Management of Overdosage
For the most up to date information on the management of RISPERDAL® overdosage, contact a certified poison control center (1-800-222-1222 or www.poison.org). Provide supportive care including close medical supervision and monitoring. Treatment should consist of general measures employed in the management of overdosage with any drug. Consider the possibility of multiple drug overdosage. Ensure an adequate airway, oxygenation, and ventilation. Monitor cardiac rhythm and vital signs. Use supportive and symptomatic measures. There is no specific antidote to RISPERDAL®.
11description
RISPERDAL® contains risperidone, an atypical antipsychotic belonging to the chemical class of benzisoxazole derivatives. The chemical designation is 3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl]-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one. Its molecular formula is C23H27FN4O2 and its molecular weight is 410.49. The structural formula is:
Risperidone is a white to slightly beige powder. It is practically insoluble in water, freely soluble in methylene chloride, and soluble in methanol and 0.1 N HCl.
RISPERDAL® Tablets are for oral administration and available in 0.5 mg (red-brown), 1 mg (white), 2 mg (orange), 3 mg (yellow), and 4 mg (green) strengths. RISPERDAL® tablets contain the following inactive ingredients: colloidal silicon dioxide, hypromellose, lactose, magnesium stearate, microcrystalline cellulose, propylene glycol, sodium lauryl sulfate, and starch (corn). The 0.5 mg, 2 mg, 3 mg, and 4 mg tablets also contain talc and titanium dioxide. The 0.5 mg tablets contain red iron oxide; the 2 mg tablets contain FD&C Yellow No. 6 Aluminum Lake; the 3 mg and 4 mg tablets contain D&C Yellow No. 10; the 4 mg tablets contain FD&C Blue No. 2 Aluminum Lake.
RISPERDAL® is also available as a 1 mg/mL oral solution. RISPERDAL® Oral Solution contains the following inactive ingredients: tartaric acid, benzoic acid, sodium hydroxide, and purified water.
RISPERDAL® M-TAB® Orally Disintegrating Tablets are available in 0.5 mg (light coral), 1 mg (light coral), 2 mg (coral), 3 mg (coral), and 4 mg (coral) strengths. RISPERDAL® M-TAB® Orally Disintegrating Tablets contain the following inactive ingredients: Amberlite® resin, gelatin, mannitol, glycine, simethicone, carbomer, sodium hydroxide, aspartame, red ferric oxide, and peppermint oil. In addition, the 2 mg, 3 mg, and 4 mg RISPERDAL® M-TAB® Orally Disintegrating Tablets contain xanthan gum.
12clinical Pharmacology
12.1Mechanism of Action
The mechanism of action of risperidone in schizophrenia is unclear. The drug's therapeutic activity in schizophrenia could be mediated through a combination of dopamine Type 2 (D2) and serotonin Type 2 (5HT2) receptor antagonism. The clinical effect from risperidone results from the combined concentrations of risperidone and its major metabolite, 9-hydroxyrisperidone (paliperidone) [see Clinical Pharmacology (12.3)]. Antagonism at receptors other than D2 and 5HT2 may explain some of the other effects of risperidone [see Clinical Pharmacology (12.1)].
12.2Pharmacodynamics
Risperidone is a monoaminergic antagonist with high affinity (Ki of 0.12 to 7.3 nM) for the serotonin Type 2 (5HT2), dopamine Type 2 (D2), α1 and α2 adrenergic, and H1 histaminergic receptors. Risperidone showed low to moderate affinity (Ki of 47 to 253 nM) for the serotonin 5HT1C, 5HT1D, and 5HT1A receptors, weak affinity (Ki of 620 to 800 nM) for the dopamine D1 and haloperidol-sensitive sigma site, and no affinity (when tested at concentrations >10-5 M) for cholinergic muscarinic or β1 and β2 adrenergic receptors.
12.3Pharmacokinetics
Absorption
Risperidone is well absorbed. The absolute oral bioavailability of risperidone is 70% (CV=25%). The relative oral bioavailability of risperidone from a tablet is 94% (CV=10%) when compared to a solution.
Pharmacokinetic studies showed that RISPERDAL® M-TAB® Orally Disintegrating Tablets and RISPERDAL® Oral Solution are bioequivalent to RISPERDAL® Tablets.
Plasma concentrations of risperidone, its major metabolite, 9-hydroxyrisperidone, and risperidone plus 9-hydroxyrisperidone are dose proportional over the dosing range of 1 to 16 mg daily (0.5 to 8 mg twice daily). Following oral administration of solution or tablet, mean peak plasma concentrations of risperidone occurred at about 1 hour. Peak concentrations of 9-hydroxyrisperidone occurred at about 3 hours in extensive metabolizers, and 17 hours in poor metabolizers. Steady-state concentrations of risperidone are reached in 1 day in extensive metabolizers and would be expected to reach steady-state in about 5 days in poor metabolizers. Steady-state concentrations of 9-hydroxyrisperidone are reached in 5–6 days (measured in extensive metabolizers).
Food Effect
Food does not affect either the rate or extent of absorption of risperidone. Thus, RISPERDAL® can be given with or without meals.
Distribution
Risperidone is rapidly distributed. The volume of distribution is 1–2 L/kg. In plasma, risperidone is bound to albumin and α1-acid glycoprotein. The plasma protein binding of risperidone is approximately 90%, and that of its major metabolite, 9-hydroxyrisperidone, is 77%. Neither risperidone nor 9-hydroxyrisperidone displaces each other from plasma binding sites. High therapeutic concentrations of sulfamethazine (100 mcg/mL), warfarin (10 mcg/mL), and carbamazepine (10mcg/mL) caused only a slight increase in the free fraction of risperidone at 10 ng/mL and 9-hydroxyrisperidone at 50 ng/mL, changes of unknown clinical significance.
Elimination
Metabolism
Risperidone is extensively metabolized in the liver. The main metabolic pathway is through hydroxylation of risperidone to 9-hydroxyrisperidone by the enzyme, CYP 2D6. A minor metabolic pathway is through N-dealkylation. The main metabolite, 9-hydroxyrisperidone, has similar pharmacological activity as risperidone. Consequently, the clinical effect of the drug results from the combined concentrations of risperidone plus 9-hydroxyrisperidone.
CYP 2D6, also called debrisoquin hydroxylase, is the enzyme responsible for metabolism of many neuroleptics, antidepressants, antiarrhythmics, and other drugs. CYP 2D6 is subject to genetic polymorphism (about 6%–8% of Caucasians, and a very low percentage of Asians, have little or no activity and are "poor metabolizers") and to inhibition by a variety of substrates and some non-substrates, notably quinidine. Extensive CYP 2D6 metabolizers convert risperidone rapidly into 9-hydroxyrisperidone, whereas poor CYP 2D6 metabolizers convert it much more slowly. Although extensive metabolizers have lower risperidone and higher 9-hydroxyrisperidone concentrations than poor metabolizers, the pharmacokinetics of risperidone and 9-hydroxyrisperidone combined, after single and multiple doses, are similar in extensive and poor metabolizers.
Excretion
Risperidone and its metabolites are eliminated via the urine and, to a much lesser extent, via the feces. As illustrated by a mass balance study of a single 1 mg oral dose of 14C-risperidone administered as solution to three healthy male volunteers, total recovery of radioactivity at 1 week was 84%, including 70% in the urine and 14% in the feces.
The apparent half-life of risperidone was 3 hours (CV=30%) in extensive metabolizers and 20 hours (CV=40%) in poor metabolizers. The apparent half-life of 9-hydroxyrisperidone was about 21 hours (CV=20%) in extensive metabolizers and 30 hours (CV=25%) in poor metabolizers. The pharmacokinetics of risperidone and 9-hydroxyrisperidone combined, after single and multiple doses, were similar in extensive and poor metabolizers, with an overall mean elimination half-life of about 20 hours.
Drug Interaction Studies
Risperidone could be subject to two kinds of drug-drug interactions. First, inhibitors of CYP 2D6 interfere with conversion of risperidone to 9-hydroxyrisperidone [see Drug Interactions (7)]. This occurs with quinidine, giving essentially all recipients a risperidone pharmacokinetic profile typical of poor metabolizers. The therapeutic benefits and adverse effects of risperidone in patients receiving quinidine have not been evaluated, but observations in a modest number (n≅70) of poor metabolizers given RISPERDAL® do not suggest important differences between poor and extensive metabolizers. Second, co-administration of known enzyme inducers (e.g., carbamazepine, phenytoin, rifampin, and phenobarbital) with RISPERDAL® may cause a decrease in the combined plasma concentrations of risperidone and 9-hydroxyrisperidone [see Drug Interactions (7)]. It would also be possible for risperidone to interfere with metabolism of other drugs metabolized by CYP 2D6. Relatively weak binding of risperidone to the enzyme suggests this is unlikely [see Drug Interactions (7)].
In vitro studies indicate that risperidone is a relatively weak inhibitor of CYP 2D6. Therefore, RISPERDAL® is not expected to substantially inhibit the clearance of drugs that are metabolized by this enzymatic pathway. In drug interaction studies, RISPERDAL® did not significantly affect the pharmacokinetics of donepezil and galantamine, which are metabolized by CYP 2D6.
In vitro studies demonstrated that drugs metabolized by other CYP isozymes, including 1A1, 1A2, 2C9, 2C19, and 3A4, are only weak inhibitors of risperidone metabolism.
Specific Populations
Renal and Hepatic Impairment
[SeeUse in Specific Populations (8.6and8.7)].
Elderly
In healthy elderly subjects, renal clearance of both risperidone and 9-hydroxyrisperidone was decreased, and elimination half-lives were prolonged compared to young healthy subjects. Dosing should be modified accordingly in the elderly patients [see Use in Specific Populations (8.5)].
Pediatric
The pharmacokinetics of risperidone and 9-hydroxyrisperidone in children were similar to those in adults after correcting for the difference in body weight.
Race and Gender Effects
No specific pharmacokinetic study was conducted to investigate race and gender effects, but a population pharmacokinetic analysis did not identify important differences in the disposition of risperidone due to gender (whether corrected for body weight or not) or race.
13nonclinical Toxicology
13.1Carcinogenesis, Mutagenesis, Impairment of Fertility
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY SECTION
Carcinogenesis
Risperidone was administered in the diet at doses of 0.63, 2.5, and 10 mg/kg for 18 months to mice and for 25 months to rats. These doses are equivalent to approximately 0.2, 0.75, and 3 times (mice) and 0.4, 1.5, and 6 times (rats) the MRHD of 16 mg/day, based on mg/m2 body surface area. A maximum tolerated dose was not achieved in male mice. There were statistically significant increases in pituitary gland adenomas, endocrine pancreas adenomas, and mammary gland adenocarcinomas. The table below summarizes the multiples of the human dose on a mg/m2 (mg/kg) basis at which these tumors occurred.
Multiples of Maximum Human Dose in mg/m2 (mg/kg) Tumor Type Species Sex Lowest Effect Level Highest No-Effect Level Pituitary adenomas mouse Female 0.75 (9.4) 0.2 (2.4) Endocrine pancreas adenomas rat Male 1.5 (9.4) 0.4 (2.4) Mammary gland adenocarcinomas mouse Female 0.2 (2.4) none rat Female 0.4 (2.4) none rat Male 6.0 (37.5) 1.5 (9.4) Mammary gland neoplasm, Total rat Male 1.5 (9.4) 0.4 (2.4)
Antipsychotic drugs have been shown to chronically elevate prolactin levels in rodents. Serum prolactin levels were not measured during the risperidone carcinogenicity studies; however, measurements during subchronic toxicity studies showed that risperidone elevated serum prolactin levels 5–6 fold in mice and rats at the same doses used in the carcinogenicity studies. An increase in mammary, pituitary, and endocrine pancreas neoplasms has been found in rodents after chronic administration of other antipsychotic drugs and is considered to be prolactin-mediated. The relevance for human risk of the findings of prolactin-mediated endocrine tumors in rodents is unclear [see Warnings and Precautions (5.6)].
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY SECTION
Mutagenesis
No evidence of mutagenic or clastogenic potential for risperidone was found in the in vitro tests of Ames gene mutation, the mouse lymphoma assay, rat hepatocyte DNA-repair assay, the chromosomal aberration test in human lymphocytes, Chinese hamster ovary cells, or in the in vivo oral micronucleus test in mice and the sex-linked recessive lethal test in Drosophila.
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY SECTION
Impairment of Fertility
Oral risperidone (0.16 to 5 mg/kg) impaired mating, but not fertility, in rat reproductive studies at doses 0.1 to 3 times the MRHD of 16 mg/day based on mg/m2 body surface area. The effect appeared to be in females, since impaired mating behavior was not noted in the male fertility study. In a subchronic study in Beagle dogs in which risperidone was administered orally at doses of 0.31 to 5 mg/kg, sperm motility and concentration were decreased at doses 0.6 to 10 times the MRHD based on mg/m2 body surface area. Dose-related decreases were also noted in serum testosterone at the same doses. Serum testosterone and sperm parameters partially recovered, but remained decreased after treatment was discontinued. A no-effect dose could not be determined in either rat or dog.
14clinical Studies
14.1Schizophrenia
Adults
Short-Term Efficacy
The efficacy of RISPERDAL® in the treatment of schizophrenia was established in four short-term (4- to 8-week) controlled trials of psychotic inpatients who met DSM-III-R criteria for schizophrenia.
Several instruments were used for assessing psychiatric signs and symptoms in these studies, among them the Brief Psychiatric Rating Scale (BPRS), a multi-li inventory of general psychopathology traditionally used to evaluate the effects of drug treatment in schizophrenia. The BPRS psychosis cluster (conceptual disorganization, hallucinatory behavior, suspiciousness, and unusual thought content) is considered a particularly useful subset for assessing actively psychotic schizophrenic patients. A second traditional assessment, the Clinical Global Impression (CGI), reflects the impression of a skilled observer, fully familiar with the manifestations of schizophrenia, about the overall clinical state of the patient. In addition, the Positive and Negative Syndrome Scale (PANSS) and the Scale for Assessing Negative Symptoms (SANS) were employed.
The results of the trials follow:
(1) In a 6-week, placebo-controlled trial (n=160) involving titration of RISPERDAL® in doses up to 10 mg/day (twice-daily schedule), RISPERDAL® was generally superior to placebo on the BPRS total score, on the BPRS psychosis cluster, and marginally superior to placebo on the SANS.(2) In an 8-week, placebo-controlled trial (n=513) involving 4 fixed doses of RISPERDAL® (2 mg/day, 6 mg/day, 10 mg/day, and 16 mg/day, on a twice-daily schedule), all 4 RISPERDAL® groups were generally superior to placebo on the BPRS total score, BPRS psychosis cluster, and CGI severity score; the 3 highest RISPERDAL® dose groups were generally superior to placebo on the PANSS negative subscale. The most consistently positive responses on all measures were seen for the 6 mg dose group, and there was no suggestion of increased benefit from larger doses.(3) In an 8-week, dose comparison trial (n=1356) involving 5 fixed doses of RISPERDAL® (1 mg/day, 4 mg/day, 8 mg/day, 12 mg/day, and 16 mg/day, on a twice-daily schedule), the four highest RISPERDAL® dose groups were generally superior to the 1 mg RISPERDAL® dose group on BPRS total score, BPRS psychosis cluster, and CGI severity score. None of the dose groups were superior to the 1 mg group on the PANSS negative subscale. The most consistently positive responses were seen for the 4 mg dose group.(4) In a 4-week, placebo-controlled dose comparison trial (n=246) involving 2 fixed doses of RISPERDAL® (4 and 8 mg/day on a once-daily schedule), both RISPERDAL® dose groups were generally superior to placebo on several PANSS measures, including a response measure (>20% reduction in PANSS total score), PANSS total score, and the BPRS psychosis cluster (derived from PANSS). The results were generally stronger for the 8 mg than for the 4 mg dose group.
Long-Term Efficacy
In a longer-term trial, 365 adult outpatients predominantly meeting DSM-IV criteria for schizophrenia and who had been clinically stable for at least 4 weeks on an antipsychotic medication were randomized to RISPERDAL® (2–8 mg/day) or to an active comparator, for 1 to 2 years of observation for relapse. Patients receiving RISPERDAL® experienced a significantly longer time to relapse over this time period compared to those receiving the active comparator.
Pediatrics
The efficacy of RISPERDAL® in the treatment of schizophrenia in adolescents aged 13–17 years was demonstrated in two short-term (6 and 8 weeks), double-blind controlled trials. All patients met DSM-IV diagnostic criteria for schizophrenia and were experiencing an acute episode at time of enrollment. In the first trial (study #1), patients were randomized into one of three treatment groups: RISPERDAL® 1–3 mg/day (n=55, mean modal dose = 2.6 mg), RISPERDAL® 4–6 mg/day (n=51, mean modal dose = 5.3 mg), or placebo (n=54). In the second trial (study #2), patients were randomized to either RISPERDAL® 0.15–0.6 mg/day (n=132, mean modal dose = 0.5 mg) or RISPERDAL® 1.5–6 mg/day (n=125, mean modal dose = 4 mg). In all cases, study medication was initiated at 0.5 mg/day (with the exception of the 0.15–0.6 mg/day group in study #2, where the initial dose was 0.05 mg/day) and titrated to the target dosage range by approximately Day 7. Subsequently, dosage was increased to the maximum tolerated dose within the target dose range by Day 14. The primary efficacy variable in all studies was the mean change from baseline in total PANSS score.
Results of the studies demonstrated efficacy of RISPERDAL® in all dose groups from 1–6 mg/day compared to placebo, as measured by significant reduction of total PANSS score. The efficacy on the primary parameter in the 1–3 mg/day group was comparable to the 4–6 mg/day group in study #1, and similar to the efficacy demonstrated in the 1.5–6 mg/day group in study #2. In study #2, the efficacy in the 1.5–6 mg/day group was statistically significantly greater than that in the 0.15–0.6 mg/day group. Doses higher than 3 mg/day did not reveal any trend towards greater efficacy.
14.2Bipolar Mania - Monotherapy
Adults
The efficacy of RISPERDAL® in the treatment of acute manic or mixed episodes was established in two short-term (3-week) placebo-controlled trials in patients who met the DSM-IV criteria for Bipolar I Disorder with manic or mixed episodes. These trials included patients with or without psychotic features.
The primary rating instrument used for assessing manic symptoms in these trials was the Young Mania Rating Scale (YMRS), an 11-li clinician-rated scale traditionally used to assess the degree of manic symptomatology (irritability, disruptive/aggressive behavior, sleep, elevated mood, speech, increased activity, sexual interest, language/thought disorder, thought content, appearance, and insight) in a range from 0 (no manic features) to 60 (maximum score). The primary outcome in these trials was change from baseline in the YMRS total score. The results of the trials follow:
(1) In one 3-week placebo-controlled trial (n=246), limited to patients with manic episodes, which involved a dose range of RISPERDAL® 1–6 mg/day, once daily, starting at 3 mg/day (mean modal dose was 4.1 mg/day), RISPERDAL® was superior to placebo in the reduction of YMRS total score.(2) In another 3-week placebo-controlled trial (n=286), which involved a dose range of 1–6 mg/day, once daily, starting at 3 mg/day (mean modal dose was 5.6 mg/day), RISPERDAL® was superior to placebo in the reduction of YMRS total score.
Pediatrics
The efficacy of RISPERDAL® in the treatment of mania in children or adolescents with Bipolar I disorder was demonstrated in a 3-week, randomized, double-blind, placebo-controlled, multicenter trial including patients ranging in ages from 10 to 17 years who were experiencing a manic or mixed episode of bipolar I disorder. Patients were randomized into one of three treatment groups: RISPERDAL® 0.5–2.5 mg/day (n=50, mean modal dose = 1.9 mg), RISPERDAL® 3–6 mg/day (n=61, mean modal dose = 4.7 mg), or placebo (n=58). In all cases, study medication was initiated at 0.5 mg/day and titrated to the target dosage range by Day 7, with further increases in dosage to the maximum tolerated dose within the targeted dose range by Day 10. The primary rating instrument used for assessing efficacy in this study was the mean change from baseline in the total YMRS score.
Results of this study demonstrated efficacy of RISPERDAL® in both dose groups compared with placebo, as measured by significant reduction of total YMRS score. The efficacy on the primary parameter in the 3–6 mg/day dose group was comparable to the 0.5–2.5 mg/day dose group. Doses higher than 2.5 mg/day did not reveal any trend towards greater efficacy.
14.3 Bipolar Mania Adjunctive Therapy with Lithium or Valproate
The efficacy of RISPERDAL® with concomitant lithium or valproate in the treatment of acute manic or mixed episodes was established in one controlled trial in adult patients who met the DSM-IV criteria for Bipolar I Disorder. This trial included patients with or without psychotic features and with or without a rapid-cycling course.
(1) In this 3-week placebo-controlled combination trial, 148 in- or outpatients on lithium or valproate therapy with inadequately controlled manic or mixed symptoms were randomized to receive RISPERDAL®, placebo, or an active comparator, in combination with their original therapy. RISPERDAL®, in a dose range of 1–6 mg/day, once daily, starting at 2 mg/day (mean modal dose of 3.8 mg/day), combined with lithium or valproate (in a therapeutic range of 0.6 mEq/L to 1.4 mEq/L or 50 mcg/mL to 120 mcg/mL, respectively) was superior to lithium or valproate alone in the reduction of YMRS total score.(2) In a second 3-week placebo-controlled combination trial, 142 in- or outpatients on lithium, valproate, or carbamazepine therapy with inadequately controlled manic or mixed symptoms were randomized to receive RISPERDAL® or placebo, in combination with their original therapy. RISPERDAL®, in a dose range of 1–6 mg/day, once daily, starting at 2 mg/day (mean modal dose of 3.7 mg/day), combined with lithium, valproate, or carbamazepine (in therapeutic ranges of 0.6 mEq/L to 1.4 mEq/L for lithium, 50 mcg/mL to 125 mcg/mL for valproate, or 4–12 mcg/mL for carbamazepine, respectively) was not superior to lithium, valproate, or carbamazepine alone in the reduction of YMRS total score. A possible explanation for the failure of this trial was induction of risperidone and 9-hydroxyrisperidone clearance by carbamazepine, leading to subtherapeutic levels of risperidone and 9-hydroxyrisperidone.14.4Irritability Associated with Autistic Disorder
Short-Term Efficacy
The efficacy of RISPERDAL® in the treatment of irritability associated with autistic disorder was established in two 8-week, placebo-controlled trials in children and adolescents (aged 5 to 16 years) who met the DSM-IV criteria for autistic disorder. Over 90% of these subjects were under 12 years of age and most weighed over 20 kg (16–104.3 kg).
Efficacy was evaluated using two assessment scales: the Aberrant Behavior Checkul (ABC) and the Clinical Global Impression - Change (CGI-C) scale. The primary outcome measure in both trials was the change from baseline to endpoint in the Irritability subscale of the ABC (ABC-I). The ABC-I subscale measured the emotional and behavioral symptoms of autism, including aggression towards others, deliberate self-injuriousness, temper tantrums, and quickly changing moods. The CGI-C rating at endpoint was a co-primary outcome measure in one of the studies.
The results of these trials are as follows:
(1) In one of the 8-week, placebo-controlled trials, children and adolescents with autistic disorder (n=101), aged 5 to 16 years, received twice daily doses of placebo or RISPERDAL® 0.5–3.5 mg/day on a weight-adjusted basis. RISPERDAL®, starting at 0.25 mg/day or0.5 mg/day depending on baseline weight (< 20 kg and ≥ 20 kg, respectively) and titrated to clinical response (mean modal dose of 1.9 mg/day, equivalent to 0.06 mg/kg/day), significantly improved scores on the ABC-I subscale and on the CGI-C scale compared with placebo.(2) In the other 8-week, placebo-controlled trial in children with autistic disorder (n=55), aged 5 to 12 years, RISPERDAL® 0.02 to 0.06 mg/kg/day given once or twice daily, starting at 0.01 mg/kg/day and titrated to clinical response (mean modal dose of 0.05 mg/kg/day, equivalent to 1.4 mg/day), significantly improved scores on the ABC-I subscale compared with placebo.
A third trial was a 6-week, multicenter, randomized, double-blind, placebo-controlled, fixed-dose study to evaluate the efficacy and safety of a lower than recommended dose of risperidone in subjects (N=96) 5 to 17 years of age with autistic disorder (defined by DSM-IV criteria) and associated irritability and related behavioral symptoms. Approximately 77% of patients were younger than 12 years of age (mean age = 9), and 88% were male. Most patients (73%) weighed less than 45 kg (mean weight = 40 kg). Approximately 90% of patients were antipsychotic-naïve before entering the study.
There were two weight-based, fixed doses of risperidone (high-dose and low-dose). The high dose was 1.25 mg per day for patients weighing 20 to < 45 kg, and it was 1.75 mg per day for patients weighing ≥ 45 kg. The low dose was 0.125 mg per day for patients weighing 20 to < 45 kg, and it was 0.175 mg per day for patients weighing ≥ 45 kg. The dose was administered once daily in the morning, or in the evening if sedation occurred.
The primary efficacy endpoint was the mean change in the Aberrant Behavior Checkul – Irritability subscale (ABC-I) score from baseline to the end of Week 6. The study demonstrated the efficacy of high-dose risperidone, as measured by the mean change in ABC-I score. It did not demonstrate efficacy for low-dose risperidone. The mean baseline ABC-I scores were 29 in the placebo group (n=35), 27 in the risperidone low-dose group (n=30), and 28 in the risperidone high-dose group (n=31). The mean changes in ABC-I scores were -3.5, -7.4, and -12.4 in the placebo, low-dose, and high-dose group respectively. The results in the high-dose group were statistically significant (p< 0.001) but not in the low-dose group (p=0.164).
Long-Term Efficacy
Following completion of the first 8-week double-blind study, 63 patients entered an open-label study extension where they were treated with RISPERDAL® for 4 or 6 months (depending on whether they received RISPERDAL® or placebo in the double-blind study). During this open-label treatment period, patients were maintained on a mean modal dose of RISPERDAL® of 1.8–2.1 mg/day (equivalent to 0.05 – 0.07 mg/kg/day).
Patients who maintained their positive response to RISPERDAL® (response was defined as ≥ 25% improvement on the ABC-I subscale and a CGI-C rating of 'much improved' or 'very much improved') during the 4–6 month open-label treatment phase for about 140 days, on average, were randomized to receive RISPERDAL® or placebo during an 8-week, double-blind withdrawal study (n=39 of the 63 patients). A pre-planned interim analysis of data from patients who completed the withdrawal study (n=32), undertaken by an independent Data Safety Monitoring Board, demonstrated a significantly lower relapse rate in the RISPERDAL® group compared with the placebo group. Based on the interim analysis results, the study was terminated due to demonstration of a statistically significant effect on relapse prevention. Relapse was defined as ≥ 25% worsening on the most recent assessment of the ABC-I subscale (in relation to baseline of the randomized withdrawal phase).
16how Supplied/storage And Handling
16.1How Supplied
RISPERDAL® (risperidone) Tablets
RISPERDAL® (risperidone) Tablets are imprinted "JANSSEN" on one side and either "Ris 0.5", "R1", "R2", "R3", or "R4" according to their respective strengths.
0.5 mg red-brown, capsule-shaped tablets: bottles of 60 NDC 50458-302-06, bottles of 500 NDC 50458-302-50, and hospital unit dose buler packs of 100 NDC 50458-302-01.
1 mg white, capsule-shaped tablets: bottles of 60 NDC 50458-300-06, bottles of 500 NDC 50458-300-50, and hospital unit dose buler packs of 100 NDC 50458-300-01.
2 mg orange, capsule-shaped tablets: bottles of 60 NDC 50458-320-06, bottles of 500 NDC 50458-320-50, and hospital unit dose buler packs of 100 NDC 50458-320-01.
3 mg yellow, capsule-shaped tablets: bottles of 60 NDC 50458-330-06, bottles of 500 NDC 50458-330-50, and hospital unit dose buler packs of 100 NDC 50458-330-01.
4 mg green, capsule-shaped tablets: bottles of 60 NDC 50458-350-06 and hospital unit dose buler packs of 100 NDC 50458-350-01.
RISPERDAL® (risperidone) Oral Solution
RISPERDAL® (risperidone) 1 mg/mL Oral Solution (NDC 50458-305-03) is supplied in 30 mL bottles with a calibrated (in milliliters) oral dosing syringe. The minimum calibrated volume is 0.25 mL, while the maximum calibrated volume is 3 mL.
RISPERDAL® M-TAB® (risperidone) Orally Disintegrating Tablets
RISPERDAL® M-TAB® (risperidone) Orally Disintegrating Tablets are etched on one side with "R0.5", "R1", "R2", "R3", or "R4" according to their respective strengths. RISPERDAL® M-TAB® Orally Disintegrating Tablets 0.5 mg, 1 mg, and 2 mg are packaged in buler packs of 4 (2 × 2) tablets. Orally Disintegrating Tablets 3 mg and 4 mg are packaged in a child-resistant pouch containing a buler with 1 tablet.
0.5 mg light coral, round, biconvex tablets: 7 buler packages (4 tablets each) per box, NDC 50458-395-28, and long-term care buler packaging of 30 tablets NDC 50458-395-30.
1 mg light coral, square, biconvex tablets: 7 buler packages (4 tablets each) per box, NDC 50458-315-28, and long-term care buler packaging of 30 tablets NDC 50458-315-30.
2 mg coral, square, biconvex tablets: 7 buler packages (4 tablets each) per box, NDC 50458-325-28.
3 mg coral, round, biconvex tablets: 28 bulers per box, NDC 50458-335-28.
4 mg coral, round, biconvex tablets: 28 bulers per box, NDC 50458-355-28.
16.2Storage and Handling
RISPERDAL® Tablets should be stored at controlled room temperature 15°–25°C (59°–77°F). Protect from light and moisture.
RISPERDAL® 1 mg/mL Oral Solution should be stored at controlled room temperature 15°–25°C (59°–77°F). Protect from light and freezing.
RISPERDAL® M-TAB® Orally Disintegrating Tablets should be stored at controlled room temperature 15°–25°C (59°–77°F).
Keep out of reach of children.
17patient Counseling Information
Advise patients using RISPERDAL oral solution to read the FDA-approved patient labeling (Instructions for Use) for RISPERDAL oral solution.
Physicians are advised to discuss the following issues with patients for whom they prescribe RISPERDAL®.
Neuroleptic Malignant Syndrome (NMS)
Counsel patients about a potentially fatal adverse reaction, Neuroleptic Malignant Syndrome (NMS), that has been reported in association with administration of antipsychotic drugs. Advise patients, family members, or caregivers to contact the healthcare provider or report to the emergency room if they experience signs and symptoms of NMS, including hyperpyrexia, muscle rigidity, altered mental status including delirium, and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmia) [see Warnings and Precautions (5.3)].
Tardive Dyskinesia
Counsel patients on the signs and symptoms of tardive dyskinesia and to contact their healthcare provider if these abnormal movements occur [see Warnings and Precautions (5.4)].
Metabolic Changes
Educate patients about the risk of metabolic changes, how to recognize symptoms of hyperglycemia and diabetes mellitus, and the need for specific monitoring, including blood glucose, lipids, and weight [see Warnings and Precautions (5.5)].
Orthostatic Hypotension
Educate patients about the risk of orthostatic hypotension and syncope, particularly at the time of initiating treatment, re-initiating treatment, or increasing the dose [see Warnings and Precautions (5.7)].
Leukopenia/Neutropenia
Advise patients with a pre-existing low WBC or a history of drug induced leukopenia/neutropenia they should have their CBC monitored while taking RISPERDAL® [see Warnings and Precautions (5.9)].
Hyperprolactinemia
Counsel patients on signs and symptoms of hyperprolactinemia that may be associated with chronic use of RISPERDAL®. Advise them to seek medical attention if they experience any of the following: amenorrhea or galactorrhea in females, erectile dysfunction or gynecomastia in males. [See Warnings and Precautions (5.6)].
Interference with Cognitive and Motor Performance
Caution patients about performing activities requiring mental alertness, such as operating hazardous machinery, or operating a motor vehicle until they are reasonably certain that RISPERDAL® therapy does not affect them adversely [see Warnings and Precautions (5.10)].
Priapism
Advise patients of the possibility of painful or prolonged penile erections (priapism). Instruct the patient to seek immediate medical attention in the event of priapism [Warnings and Precautions (5.13)].
Heat Exposure and Dehydration
Counsel patients regarding appropriate care in avoiding overheating and dehydration [see Warnings and Precautions (5.14)].
Phenylketonurics
Inform patients with Phenylketonuria and caregivers that RISPERDAL® M-TAB® Orally Disintegrating Tablets contain phenylalanine. Phenylalanine is a component of aspartame. Each 4 mg RISPERDAL® M-TAB® Orally Disintegrating Tablet contains 0.84 mg phenylalanine; each 3 mg RISPERDAL® M-TAB® Orally Disintegrating Tablet contains 0.63 mg phenylalanine; each 2 mg RISPERDAL® M-TAB® Orally Disintegrating Tablet contains 0.42 mg phenylalanine; each 1 mg RISPERDAL® M-TAB® Orally Disintegrating Tablet contains 0.28 mg phenylalanine; and each 0.5 mg RISPERDAL® M-TAB® Orally Disintegrating Tablet contains 0.14 mg phenylalanine [seeWarnings and Precautions (5.15)].
Concomitant Medication
Advise patients to inform their healthcare providers if they are taking, or plan to take any prescription or over-the-counter drugs, as there is a potential for interactions [see Drug Interactions (7)].
Alcohol
Advise patients to avoid alcohol while taking RISPERDAL® [see Drug Interactions (7.2)].
Pregnancy
Advise patients to notify their healthcare provider if they become pregnant or intend to become pregnant during treatment with RISPERDAL®. Advise patients that RISPERDAL® may cause extrapyramidal and/or withdrawal symptoms in a neonate. Advise patients that there is a pregnancy registry that monitors pregnancy outcomes in women exposed to RISPERDAL® during pregnancy [see Use in Specific Populations (8.1)].
Lactation
Advise breastfeeding women using RISPERDAL® to monitor infants for somnolence, failure to thrive, jitteriness, and extrapyramidal symptoms (tremors and abnormal muscle movements) and to seek medical care if they notice these signs [see Use in Specific Populations (8.2)].
Infertility
Advise females of reproductive potential that RISPERDAL® may impair fertility due to an increase in serum prolactin levels. The effects on fertility are reversible [see Use in Specific Populations (8.3)].
RISPERDAL ® Tablets Active ingredient is made in IrelandFinished product is manufactured by:Janssen Ortho LLCGurabo, Puerto Rico 00778
RISPERDAL ® Oral Solution Finished product is manufactured by:Janssen Pharmaceutica NVBeerse, Belgium
RISPERDAL ® M-TAB ® Orally Disintegrating Tablets Active ingredient is made in IrelandFinished product is manufactured by:Janssen Ortho, LLCGurabo, Puerto Rico 00778
RISPERDAL® Tablets, RISPERDAL® M-TAB® Orally Disintegrating Tablets, and RISPERDAL® Oral Solution are manufactured for:Janssen Pharmaceuticals, Inc.Titusville, NJ 08560
© 2007 Janssen Pharmaceutical Companies
Instructions For Use
RISPERDAL® (RISS-per-dal) (risperidone)Oral Solution
Read these Instructions for Use before you start using RISPERDAL Oral Solution and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment.
Important information you need to know before taking RISPERDAL Oral Solution:
- Take RISPERDAL Oral Solution exactly as your healthcare provider tells you to take it.
- Each 1 mL contains 1 mg of RISPERDAL Oral Solution.
- Ask your healthcare provider or pharmacist to show you how to measure your prescribed dose using the oral dosing syringe.
- Always use the oral dosing syringe that comes with RISPERDAL Oral Solution. Contact your healthcare provider or pharmacist if you lose or damage the oral dosing syringe, or if your carton does not come with one.
- RISPERDAL Oral Solution can be taken directly from the oral dosing syringe or mixed with water, coffee, orange juice, or low-fat milk. Do not mix RISPERDAL Oral Solution with cola or tea.
Each RISPERDAL Oral Solution carton contains:
- 1 bottle of RISPERDAL Oral Solution
- 1 oral dosing syringe
Gather and check supplies:
- Gather the RISPERDAL Oral Solution bottle and oral dosing syringe.
- Check the expiration date on the bottle. Do not use the bottle of RISPERDAL Oral Solution if the expiration date has passed.
- Check your dose in mLs as prescribed by your healthcare provider. Find this mL marking on the plunger of the oral dosing syringe. If your dose is more than 3 mL, you will need to divide your dose. Follow the instructions given to you by your healthcare provider or pharmacist on how to divide your dose.
Preparing a dose of RISPERDAL Oral Solution:
Step 1. Place the RISPERDAL Oral Solution bottle on a flat surface. Push down on the cap while turning it to the left (counterclockwise) to open the bottle. 
Step 2. Push the plunger of the oral dosing syringe all the way down. 
Step 3. With the bottle in an upright position, fully insert the oral dosing syringe into the opening of the bottle. 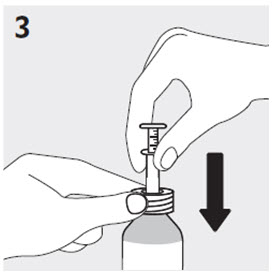
Step 4. Withdraw the prescribed dose of RISPERDAL Oral Solution from the bottle. Hold down the barrel of the oral dosing syringe with one hand. With your other hand, slowly pull the plunger up until you reach the mL markings on the plunger for the prescribed dose. 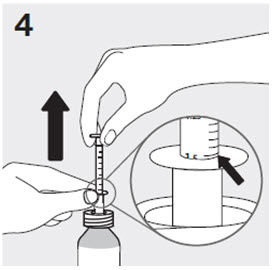
Step 5. Remove the oral dosing syringe from the bottle by holding the outer barrel and pulling straight up. Be careful not to push down on the plunger during this step. Check the oral dosing syringe for air bubbles. If you see air bubbles, slowly push the plunger all the way down to return the oral solution into the bottle. Then repeat Step 4 to withdraw the prescribed dose. 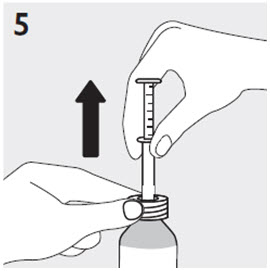
Step 6. RISPERDAL Oral Solution can be mixed with a drink or taken directly from the oral dosing syringe.
- Mix the dose of RISPERDAL Oral Solution with water, coffee, orange juice, or low-fat milk. Stir well and drink all of the mixture right away to ensure the full dose is taken. See Figure 6a. Do not mix RISPERDAL Oral Solution with cola or tea.
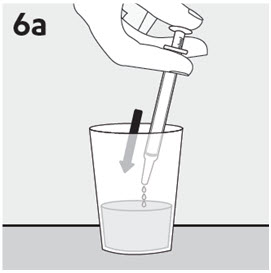
Or
- To take the RISPERDAL Oral Solution dose directly from the oral dosing syringe, place the tip of the oral dosing syringe into the mouth and toward the cheek. Slowly push the plunger all the way down to gently release all of the medicine in the oral dosing syringe. Do not squirt or forcefully push the medicine into the back of the throat. See Figure 6b .
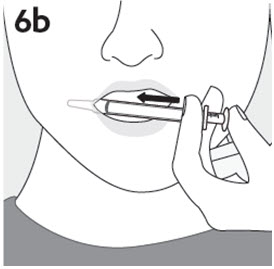
Step 7. Place the cap back on the RISPERDAL Oral Solution bottle and turn the cap to the right (clockwise) to close the bottle. 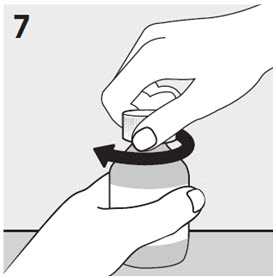
Step 8. Rinse the oral dosing syringe with water after each use. Do not throw away the oral dosing syringe.
- Remove the plunger from the oral dosing syringe barrel.
- Rinse the oral dosing syringe barrel and plunger with water and let them air dry.
- When the oral dosing syringe barrel and plunger are dry, put the plunger back into the oral dosing syringe barrel for the next use.
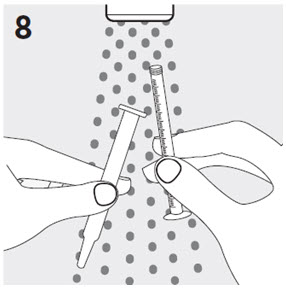
Storing RISPERDAL Oral Solution:
- Store RISPERDAL Oral Solution at room temperature between 59°F to 77°F (15°C to 25°C).
- Do not freeze RISPERDAL Oral Solution. Protect from light.
- Keep RISPERDAL Oral Solution and all medicines out of the reach of children.
Manufactured by:Janssen Pharmaceutica NVBeerse, Belgium
Manufactured for:Janssen Pharmaceuticals, Inc.Titusville, NJ, 08560
This Instructions for Use has been approved by the U.S. Food and Drug Administration.Issued: 3/2022
Instrucciones De Uso
RISPERDAL® (RISS-per-dal) (risperidona)Solución oral
Lea estas Instrucciones de uso antes de comenzar a usar RISPERDAL Solución oral y cada vez que renueve su receta. Es posible que este material contenga información nueva. Esta información no reemplaza la consulta con su proveedor de atención médica acerca de su enfermedad o tratamiento.
Información importante que debe saber antes de tomar RISPERDAL Solución oral:
- Tome RISPERDAL Solución oral exactamente como se lo indica su proveedor de atención médica.
- Cada 1 ml contiene 1 mg de RISPERDAL Solución oral.
- PÃdale a su proveedor de atención médica o farmacéutico que le muestre cómo medir la dosis recetada con la jeringa de dosificación oral.
- Utilice siempre la jeringa de dosificación oral que viene con RISPERDAL Solución oral. ComunÃquese con su proveedor de atención médica o farmacéutico si pierde o daña la jeringa de dosificación oral, o si su caja no viene con una.
- RISPERDAL Solución oral se puede tomar directamente de la jeringa de dosificación oral o se puede mezclar con agua, café, jugo de naranja o leche con bajo contenido de grasa. No mezcle RISPERDAL Solución oral con refrescos de cola o té.
Cada caja de RISPERDAL Solución oral contiene lo siguiente:
- 1 frasco de RISPERDAL Solución oral
- 1 jeringa de dosificación oral
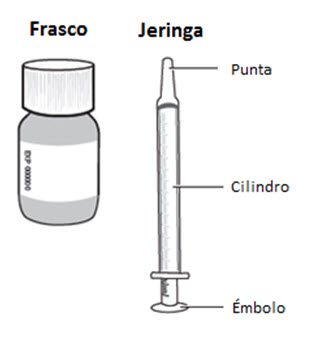
Reúna y verifique los suministros:
- Reúna el frasco de RISPERDAL Solución oral y la jeringa de dosificación oral.
- Consulte la fecha de caducidad en el frasco. No utilice el frasco de RISPERDAL Solución oral si ha pasado la fecha de caducidad.
- Verifique su dosis en ml según lo prescrito por su proveedor de atención médica. Busque esta marca de ml en el émbolo de la jeringa de dosificación oral. Si su dosis supera los 3 ml, deberá dividir la dosis. Siga las instrucciones que le dé su proveedor de atención médica o farmacéutico sobre cómo dividir su dosis.
Preparación de una dosis de RISPERDAL Solución oral:
Paso 1. Coloque el frasco de RISPERDAL Solución oral sobre una superficie plana. Empuje hacia abajo la tapa mientras la gira hacia la izquierda (en sentido antihorario) para abrir el frasco. 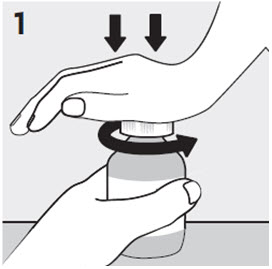
Paso 2. Empuje el émbolo de la jeringa de dosificación oral hasta el fondo. 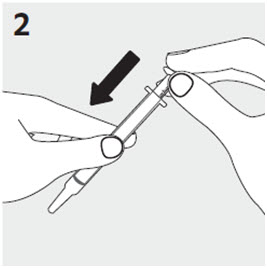
Paso 3. Con el frasco en posición vertical, inserte completamente la jeringa de dosificación oral en la abertura del frasco. 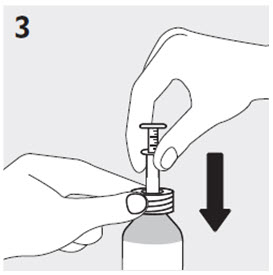
Paso 4. Extraiga la dosis prescrita de RISPERDAL Solución oral del frasco. Sostenga el cilindro de la jeringa de dosificación oral con una mano. Con la otra mano, tire lentamente del émbolo hacia arriba hasta que alcance las marcas de ml en el émbolo para la dosis prescrita. 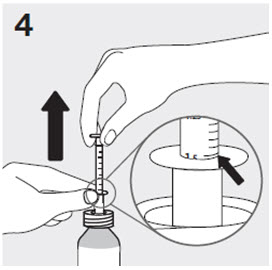
Paso 5. Retire la jeringa de dosificación oral del frasco sujetando el cilindro exterior y tirando hacia arriba. Tenga cuidado de no empujar hacia abajo el émbolo durante este paso. Compruebe que no haya burbujas de aire en la jeringa de dosificación oral. Si ve burbujas de aire, empuje lentamente el émbolo hasta el fondo para devolver la solución oral al frasco. Luego repita el Paso 4 para retirar la dosis prescrita. 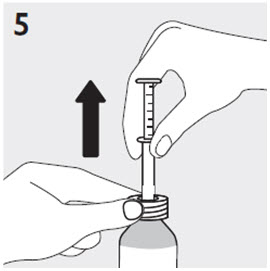
Paso 6. RISPERDAL Solución oral se puede mezclar con una bebida o se puede tomar directamente de la jeringa de dosificación oral.
- Mezcle la dosis de RISPERDAL Solución oral con agua, café, jugo de naranja o leche con bajo contenido de grasa. Revuelva bien y beba toda la mezcla de inmediato para asegurarse de tomar la dosis completa. Consulte la Figura 6a. No mezcle RISPERDAL Solución oral con refrescos de cola o té.
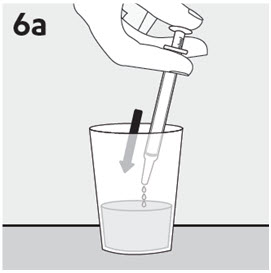
O
- Para tomar la dosis de RISPERDAL Solución oral directamente de la jeringa de dosificación oral, coloque la punta de la jeringa de dosificación oral en la boca y hacia la mejilla. Empuje lentamente el émbolo hasta el fondo para liberar suavemente todo el medicamento en la jeringa de dosificación oral. No rocÃe ni empuje con fuerza el medicamento en la parte posterior de la garganta. Consulte la Figura 6b .
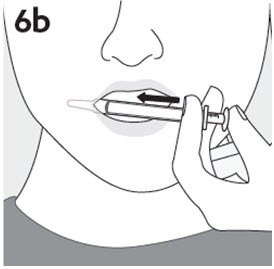
Paso 7. Vuelva a colocar la tapa en el frasco de RISPERDAL Solución oral y gÃrela hacia la derecha (en el sentido de las agujas del reloj) para cerrar el frasco. 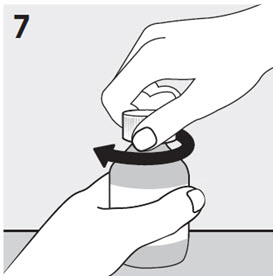
Paso 8. Enjuague la jeringa de dosificación oral con agua después de cada uso. No deseche la jeringa de dosificación oral.
- Retire el émbolo del cilindro de la jeringa de dosificación oral.
- Enjuague el cilindro de la jeringa de dosificación oral con agua y déjela secar al aire.
- Cuando el cilindro de la jeringa de dosificación oral y el émbolo estén secos, vuelva a colocar el émbolo en el cilindro de la jeringa de dosificación oral para el próximo uso.
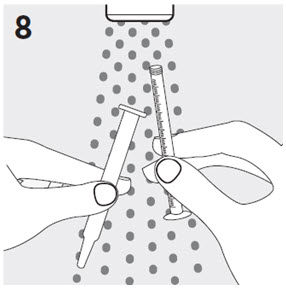
Almacenamiento de RISPERDAL Solución oral
- Almacene RISPERDAL Solución oral a temperatura ambiente entre 59 °F y 77 °F (de 15 °C a 25 °C).
- No congelar RISPERDAL Solución oral. Proteger de la luz.
- Mantenga RISPERDAL Solución oral y todos los medicamentos fuera del alcance de los niños.
Fabricado por:Janssen Pharmaceutica NVBeerse, Bélgica
Fabricado para:Janssen Pharmaceuticals, Inc. Titusville, NJ, 08560
Estas Instrucciones de uso han sido aprobadas por la Administración de Alimentos y Medicamentos (FDA) de los EE. UU.
Revisado: 3/2022
Package Label.principal Display Panel
PRINCIPAL DISPLAY PANEL - 0.5 mg Bottle Label
NDC 50458-302-06
RISPERDAL ® (risperiDONE)TABLETS
0.5 mg
Each tablet contains: Risperidone 0.5 mg
Keep out of reach of children.
Rx only
60 Tablets

Package Label.principal Display Panel
PRINCIPAL DISPLAY PANEL - 1 mg Bottle Label
NDC 50458-300-06
RISPERDAL ® (risperiDONE)TABLETS
1 mg
Each tablet contains: Risperidone 1 mg
Keep out of reach of children.
Rx only
60 Tablets

Package Label.principal Display Panel
PRINCIPAL DISPLAY PANEL - 2 mg Bottle Label
NDC 50458-320-06
RISPERDAL ® (risperiDONE)TABLETS
2 mg
Each tablet contains: Risperidone 2 mg
Keep out of reach of children.
Rx only
60 Tablets

Package Label.principal Display Panel
PRINCIPAL DISPLAY PANEL - 3 mg Bottle Label
60 TABLETSNDC 50458-330-06
RISPERDAL ® (risperiDONE)TABLETS
3 mg
Each tablet contains: Risperidone 3 mg
Keep out of reach of children.
Rx only
60 Tablets

Package Label.principal Display Panel
PRINCIPAL DISPLAY PANEL - 4 mg Bottle Label
60 TABLETSNDC 50458-350-06
RISPERDAL ® (risperiDONE)TABLETS
4 mg
Each tablet contains: Risperidone 4 mg
Keep out of reach of children.
Rx only
60 Tablets

Package Label.principal Display Panel
PRINCIPAL DISPLAY PANEL - 0.5 mg Tablet Carton
NDC 50458-395-2828 TABLETS
Risperdal ®M-TAB ® risperiDONE Orally Disintegrating Tablets
0.5 mg
Rx Only Each tablet contains:0.5 mg risperidone
Buler pack7 cards of 4 tablets
janssen
Package Label.principal Display Panel
PRINCIPAL DISPLAY PANEL - 1 mg Tablet Carton
NDC 50458-315-2828 TABLETS
Risperdal ®M-TAB ® risperiDONE Orally Disintegrating Tablets
1 mg
Rx Only Each tablet contains:1 mg risperidone
Buler pack7 cards of 4 tablets
janssen
Package Label.principal Display Panel
PRINCIPAL DISPLAY PANEL - 2 mg Tablet Carton
NDC 50458-325-2828 TABLETS
Risperdal ®M-TAB ® risperiDONE Orally Disintegrating Tablets
2 mg
Rx Only Each tablet contains:2 mg risperidone
Buler pack7 cards of 4 tablets
janssen
Package Label.principal Display Panel
PRINCIPAL DISPLAY PANEL - 3 mg Tablet Carton
NDC 50458-335-28
Risperdal ®M-TAB ® risperiDONE Orally Disintegrating Tablets
Phenylketonurics: Contains 0.63 mg phenylalanine (a component of aspartame) per 3 mg tablet Each tablet contains: 3 mg risperidone
Rx only
Dosage: For information or use, see accompanying full Prescribing Information.
Product of Ireland
Manufactured by:Janssen Ortho, LLCGurabo, PR 00778
Manufactured for:Janssen Pharmaceuticals, Inc.Titusville, NJ 08560
janssen

Package Label.principal Display Panel
PRINCIPAL DISPLAY PANEL - 4 mg Tablet Carton
NDC 50458-355-28
Risperdal ®M-TAB ® risperiDONE Orally Disintegrating Tablets
Phenylketonurics: Contains 0.84 mg phenylalanine (a component of aspartame) per 4 mg tablet Each tablet contains: 4 mg risperidone
Rx only
Dosage: For information or use, see accompanying full Prescribing Information.
Product of Ireland
Manufactured by:Janssen Ortho, LLCGurabo, PR 00778
Manufactured for:Janssen Pharmaceuticals, Inc.Titusville, NJ 08560
janssen

Package Label.principal Display Panel
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton
NDC 50458-305-03 30 mL
RISPERDAL ® (risperiDONE)ORAL SOLUTION
1 mg/mL
Each 1 mL contains: 1 mg of risperidonein an aqueous solution.
janssen
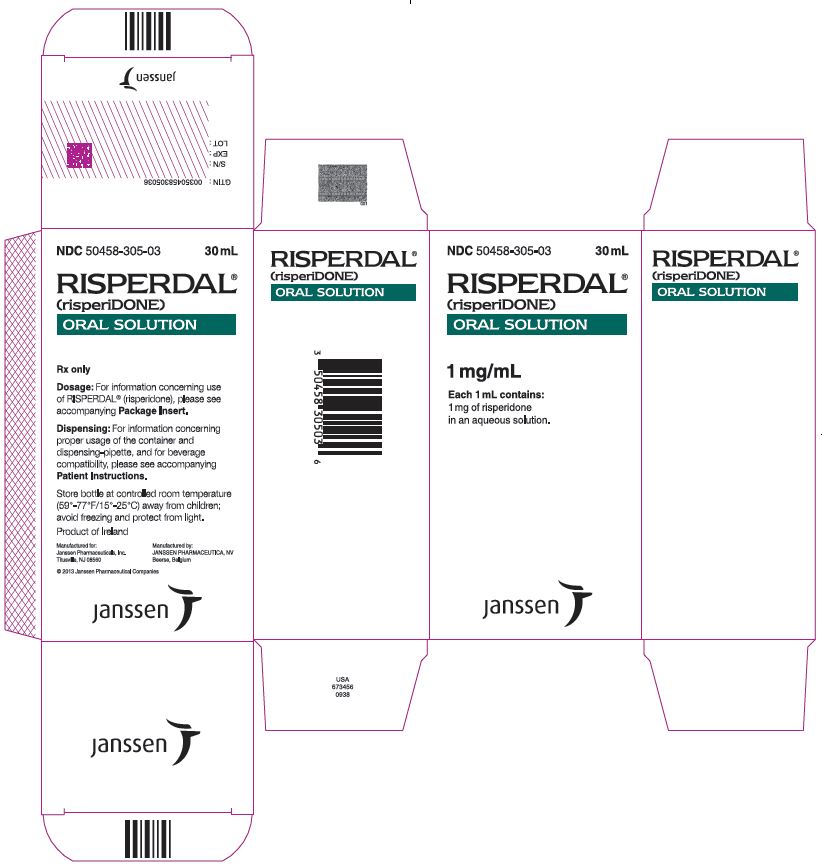
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site


