Ryzumvi Dailymed
Generic: phentolamine mesylate is used for the treatment of Intracranial Arteriosclerosis Coronary Artery Disease Extravasation of Diagnostic and Therapeutic Materials Hypertension, Malignant Kidney Diseases Necrosis Pheochromocytoma
Go PRO for all pill images
1 Indications And Usage
Ryzumvi is indicated for the treatment of pharmacologically-induced mydriasis produced by adrenergic agonists (e.g., phenylephrine) or parasympatholytic (e.g., tropicamide) agents.
Ryzumvi is an alpha adrenergic blocker indicated for the treatment of pharmacologically-induced mydriasis produced by adrenergic agonists (e.g., phenylephrine) or parasympatholytic (e.g., tropicamide) agents. (1 )
2 Dosage And Administration
Adults and pediatric patients aged 12 years or older: Instill 1 or 2 drops in each dilated eye following the completion of the ophthalmic examination or procedure. If 2 drops are instilled, the second drop should be administered 5 minutes after the first drop.
Pediatric patients aged 3 to 11 years: Instill 1 drop in each dilated eye following the completion of the ophthalmic examination or procedure.
One single-patient-use vial can be used to dose each dilated eye. Discard the single-patient-use vial immediately after use.
- • Adults and pediatric patients aged 12 years and older: Instill 1 to 2 drops in each dilated eye following the completion of the ophthalmic examination or procedure to reverse mydriasis. (
2 )- • Pediatric patients aged 3 to 11 years: Instill 1 drop in each dilated eye following the completion of the ophthalmic examination or procedure to reverse mydriasis. (
2 )
3 Dosage Forms And Strengths
Ophthalmic solution: clear and colorless solution containing phentolamine 0.75% in a single-patient-use vial.
Ophthalmic solution: phentolamine 0.75% in a single-patient-use vial. (3 )
4 Contraindications
None.
None. (4 )
5 Warnings And Precautions
Uveitis: Ryzumvi is not recommended to be used in patients with active ocular inflammation. (5.1 )
5.1 Uveitis
Ryzumvi is not recommended when active ocular inflammation (e.g. iritis) is present because adhesions (synechiae) may form between the iris and the lens.
5.2 Potential for Eye Injury or Contamination
To avoid the potential for eye injury or contamination, care should be taken to avoid touching the vial tip to the eye or to any other surface.
5.3 Use with Contact Lenses
Contact lens wearers should be advised to remove their lenses prior to the instillation of Ryzumvi and wait 10 minutes after dosing before reinserting their contact lenses.
6 Adverse Reactions
The most common adverse reactions that have been reported are instillation site discomfort (16%), conjunctival hyperemia (12%), and dysgeusia (6%). (6.1 )
To report SUSPECTED ADVERSE REACTIONS, contact Ocuphire Pharma, Inc. at 1-877-EYE-0123 or FDA at 1-800-FDA-1088 orwww.fda.gov/medwatch.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Ryzumvi was evaluated in 642 subjects in clinical trials across various subject populations. The most common ocular adverse reactions reported in >5% of subjects were instillation site discomfort including pain, stinging, and burning (16%) and conjunctival hyperemia (12%). The only non-ocular adverse reaction reported in >5% of subjects was dysgeusia (6%).
8 Use In Specific Populations
8.1 Pregnancy
There are no available data with Ryzumvi administration in pregnant women to inform a drug-associated risk. In animal toxicology studies, when phentolamine was administered orally to pregnant mice and rats during the period of organogenesis skeletal immaturity and decreased growth was observed in the offspring at doses at least 24-times the recommended clinical dose. Additionally, a lower rate of implantation was seen in pregnant rats treated with phentolamine administered at least 60-times the recommended clinical dose. No malformations or embryofetal deaths were observed in the offspring of pregnant mice, rats, and rabbits administered phentolamine during the period of organogenesis at doses of at least 24-, 60-, and 20-times, respectively, the recommended clinical dose (see Data). Ryzumvi should only be used during pregnancy if the potential benefit justifies the potential risk to the fetus.
Animal Data
Oral administration of phentolamine to pregnant rats and mice at doses at least 24-times the recommended clinical dose (based on a body weight per surface area (mg/m2) comparison with a 60-kg human) resulted in slightly decreased growth and slight skeletal immaturity of the fetuses. Immaturity was manifested by increased incidence of incomplete or unossified calcanei and phalangeal nuclei of the hind limb and of incompletely ossified sternebrae. At oral phentolamine doses at least 60-times the recommended clinical dose (based on a mg/m2 comparison with a 60-kg human), a slightly lower rate of implantation was found in rats. Phentolamine did not affect embryonic or fetal development in rabbits at oral doses at least 20-times the recommended dose (based on a mg/m2 comparison with a 60-kg human). No malformations or embryofetal deaths were observed in the rat, mouse or rabbit studies.
8.2 Lactation
There is no information regarding the presence of phentolamine in human milk, the effects on the breastfed infants, or the effects on milk production during lactation to inform risk of phentolamine ophthalmic solution 0.75% to an infant. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Ryzumvi and any potential adverse effects on the breastfed child from Ryzumvi.
8.4 Pediatric Use
The safety and effectiveness of Ryzumvi have been established in pediatric patients aged 3 to 17 years. No overall differences have been observed between pediatric and adult subjects [see Clinical Studies (14)].
8.5 Geriatric Use
No overall differences in safety and effectiveness have been observed between elderly and younger adult subjects.
10 Overdosage
No deaths due to acute poisoning with phentolamine have been reported. Overdosage with parenterally administered phentolamine is characterized chiefly by cardiovascular disturbances, such as arrhythmias, tachycardia, hypotension, and possibly shock. In addition, the following might occur: excitation, headache, sweating, visual disturbances, nausea, vomiting, diarrhea, or hypoglycemia. There is no specific antidote; treatment consists of appropriate monitoring and supportive care. Substantial decreases in blood pressure or other evidence of shock-like conditions should be treated vigorously and promptly.
11 Description
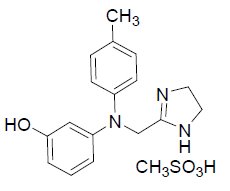
Ryzumvi (phentolamine ophthalmic solution) 0.75% is a sterile, clear and colorless solution for topical ophthalmic use containing 1% phentolamine mesylate (equivalent to 0.75% phentolamine). The product does not contain an anti-microbial preservative. The chemical name of phentolamine mesylate is 3-[[(4,5-dihydro-1H-imidazol-2-yl)methyl](4-methylphenyl)amino]phenol; methanesulfonic acid (parent phentolamine: [3-[[(4,5-dihydro-1H-imidazol-2-yl)methyl](4-methylphenyl)amino]phenol]) and the molecular formula is C18H23N3O4S (parent C17H19N3O). The molecular weight of phentolamine mesylate is 377.46 and the chemical structure is:
Each mL of Ryzumvi contains phentolamine mesylate 10 mg as the active ingredient (equivalent to 7.5 mg phentolamine as the free base). Inactive ingredients are: mannitol, sodium acetate trihydrate, and water for injection. Hydrochloric acid and/or sodium hydroxide are added to adjust pH (4.5 to 5.5), and the solution is overlaid with nitrogen.
12 Clinical Pharmacology
12.1 Mechanism of Action
Ryzumvi is a relatively non-selective alpha-1 and alpha-2 adrenergic antagonist. Dilation of the pupil is primarily controlled by the radial iris dilator muscles surrounding the pupil; these muscles are activated by the alpha-1 adrenergic receptors. Phentolamine reversibly binds to these receptors on the iris dilator muscle, thereby reducing pupil diameter. Phentolamine directly antagonizes the mydriatic effect of an α-1 adrenergic agonist, and indirectly reverses mydriasis induced by muscarinic antagonist effects on the iris sphincter muscle.
12.2 Pharmacodynamics
The onset of action after administration of Ryzumvi generally occurs in 30 minutes, with the maximal effect seen in 60 to 90 minutes, and the effect lasting at least 24 hours.
12.3 Pharmacokinetics
Phentolamine systemic exposure was evaluated in a Phase 3 trial (MIRA-3) following topical ocular administration of a total of 3 drops, each of 0.03 mL, of phentolamine ophthalmic solution 0.75%. The peak concentration levels are achieved between 15 minutes and 1 hour after dosing with the median value of 0.45 ng/mL.
13 Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies with Ryzumvi have not been conducted.
Phentolamine was not mutagenic in the in-vitro bacterial reverse mutation (Ames) assay. In the in-vitro chromosomal aberration study in Chinese hamster ovary cells, numerical aberrations were slightly increased after a 4-hour exposure to phentolamine without metabolic activation, and structural aberrations were slightly increased after a 4-hour exposure to phentolamine with metabolic activation only at the highest concentrations tested, but neither numerical nor structural aberrations were increased after a 20-hour exposure without metabolic activation. Phentolamine was not clastogenic in two in-vivo mouse micronucleus assays.
The effect of phentolamine on female fertility has not been studied. Male rats treated with oral phentolamine for nine weeks (four weeks prior to mating, 3 weeks during the mating period and 2 weeks after mating) were mated with untreated females. At doses up to 648-times human therapeutic exposure levels at the Cmax, no adverse effects on male fertility parameters or on reproductive parameters in the untreated females mated with the treated males were observed.
14 Clinical Studies
The efficacy of Ryzumvi for the reversal of mydriasis was demonstrated in two, randomized, double-masked, vehicle-controlled trials; MIRA-2 (NCT#04620213) and MIRA-3 (NCT#05134974). A total of 553 subjects, aged 12 to 80 years, who had mydriasis induced by instillation of phenylephrine or tropicamide or a combination of hydroxyamphetamine hydrobromide and tropicamide (Paremyd) were randomized. Subjects with light and dark irides were included in both trials. Two drops (study eye) or one drop (fellow eye) of Ryzumvi or placebo (vehicle) were administered one hour after instillation of the mydriatic agent. The percentage of subjects with study eyes returning to ≤0.2 mm from baseline pupil diameter was statistically significantly greater (p<0.01) at all time points measured from 60 minutes through 24 hours in the Ryzumvi group compared with the placebo (vehicle) group across both of the MIRA-2 and MIRA-3 trials (see Figure 1).
Figure 1. Percentage of subjects with study eyes returning to ≤0.2 mm from baseline pupil diameter by time point in the MIRA-2 and MIRA-3 Trials
The efficacy of MIRA-2 and MIRA-3 also showed that the change from maximum pupil dilation in study eyes and fellow eyes was statistically significantly different between the Ryzumvi-treated group and the placebo-treated group at all time points from 60 minutes through 24 hours post-treatment (p<0.01). Pupil size at 24 hours was 1 mm smaller than baseline. These results were consistent regardless of whether phenylephrine or tropicamide/Paremyd were used as mydriatic agents (Figure 2, Figure 3; respectively).
Figure 2: Pupil Dilation by Time Point with Phenylephrine as Mydriatic Agent in MIRA-2 and MIRA-3 Trials (mITT population)
Figure 3: Pupil Dilation by Time Point with Tropicamide or Paremyd as Mydriatic Agent in MIRA-2 and MIRA-3 trials (mITT Population)
The efficacy of Ryzumvi was similar for all age ranges including pediatric subjects aged 3 to 17 years. Pediatric subjects aged 12 to 17 years (n=27) were treated in MIRA-2 and MIRA-3 and pediatric subjects, aged 3 to 11 years (n=11) were treated in MIRA-4, (NCT#05223478).
16 How Supplied/storage And Handling
Ryzumvi (phentolamine ophthalmic solution) 0.75% is supplied as a sterile, clear, and colorless solution for topical ophthalmic use contained in a translucent, low-density polyethylene, single-patient-use vial with a 0.31 mL fill. One strip of 5 single-patient-use vials is packaged into a foil pouch, with 6 foil pouches in a carton. One single-patient-use vial should be dispensed for each patient, and it can be used to dose both eyes.
Carton of 30 single-patient-use vials – NDC-83368-075-30
Storage and Handling:
Store refrigerated at 2°C to 8°C (36°F to 46°F), not to exceed the expiration date printed on the carton and pouch. Protect from freezing.
After opening the foil pouch, the product may be stored at 25ºC (68°F to 77ºF) and should be used within 14 days, not to exceed the expiration date printed on the vial. The single-patient-use vial once opened should be discarded immediately after use.
Distributed by:Ocuphire Pharma, Inc.Farmington Hills, MI 48335
Marketed by:Oyster Point Pharma, Inc., a Viatris companyPrinceton, NJ 08540
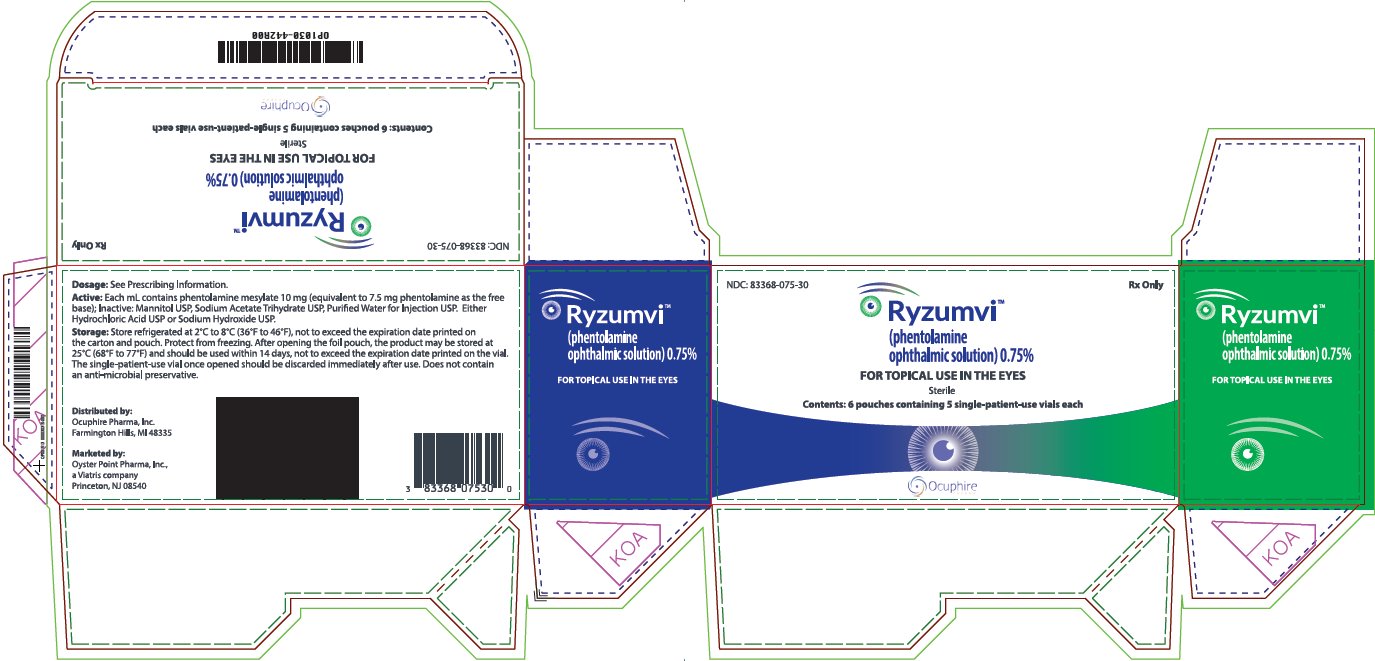
Principal Display Panel 0.75%
NDC: 83368-075-30 Rx Only
Ryzumvi™(phentolamineophthalmic solution) 0.75%
FOR TOPICAL USE IN THE EYES
Sterile
Contents: 6 pouches containing 5 single-patient-use vials each
Dosage: See Prescribing Information.
Active: Each ml contains phentolamine mesylate 10 mg (equivalent to 7.5 mg phentolamine as the free base); Inactive: Mannitol USP, Sodium Acetate Trihydrate USP, Purified Water for Injection USP. Either Hydrochloric Acid USP or Sodium Hydroxide USP.
Storage: Store refrigerated at 2°c to 8°C (36°F to 46°F), not to exceed the expiration date printed on the carton and pouch. Protect from freezing. After opening the foil pouch, the product may be stored at 25°C (68°F to 77°F) and should be used within 14 days, not to exceed the expiration date printed on the vial.
The single-patient-use vial once opened should be discarded immediately after use. Does not contain an anti-microbial preservative.
Distributed by:Ocuphire Pharma, Inc. Farmington Hills, Ml 48335
Marketed by: Oyster Point Pharma, Inc.,a Viatris companyPrinceton, NJ 08540
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site




