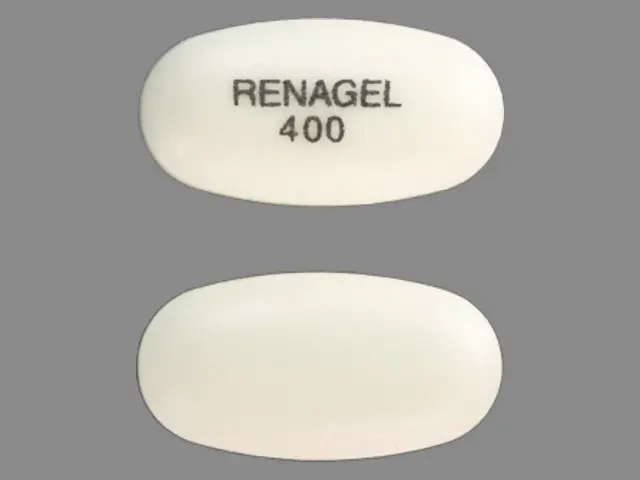
Renagel (sevelamer hydrochloride 400 mg) Dailymed
Generic: sevelamer hydrochloride
IMPRINT: RENAGEL 400
SHAPE: oval
COLOR: white
All Imprints
sevelamer hydrochloride 800 mg - renagel 800 oval white
sevelamer hydrochloride 400 mg - renagel 400 oval white
Go PRO for all pill images
1 Indications And Usage
Renagel ®is indicated for the control of serum phosphorus in patients with chronic kidney disease (CKD) on dialysis. The safety and efficacy of Renagel in CKD patients who are not on dialysis have not been studied.
- Renagel ®is a phosphate binder indicated for the control of serum phosphorus in patients with chronic kidney disease on dialysis. (
1 )
2 Dosage And Administration
- Starting dose is one or two 800 mg tablets three times per day with meals. (
2 )- Adjust by one tablet per meal in two-week intervals as needed to obtain serum phosphorus target (3.5 to 5.5 mg/dL). (
2 )
Patients Not Taking a Phosphate Binder. The recommended starting dose of Renagel is 800 to 1600 mg, which can be administered as one or two 800 mg Renagel tablets with meals based on serum phosphorus level. Table 1 provides recommended starting doses of Renagel for patients not taking a phosphate binder.
Table 1: Starting Dose for Dialysis Patients Not Taking a Phosphate Binder Serum Phosphorus Renagel 800 mg >5.5 and <7.5 mg/dL 1 tablet three times daily with meals ≥7.5 and <9.0 mg/dL 2 tablets three times daily with meals ≥9.0 mg/dL 2 tablets three times daily with meals
Patients Switching from Calcium Acetate. In a study in 84 CKD patients on hemodialysis, a similar reduction in serum phosphorus was seen with equivalent doses (approximately mg for mg) of Renagel and calcium acetate. Table 2 gives recommended starting doses of Renagel based on a patient's current calcium acetate dose.
Table 2: Starting Dose for Dialysis Patients Switching from Calcium Acetate to Renagel Calcium Acetate 667 mg (Tablets per meal) Renagel 800 mg (Tablets per meal) 1 tablet 1 tablet 2 tablets 2 tablets 3 tablets 3 tablets
Dose Titration for All Patients Taking Renagel. Adjust dosage based on the serum phosphorus concentration with a goal of lowering serum phosphorus to 5.5 mg/dL or less. Increase or decrease by one tablet per meal at two-week intervals as necessary. Table 3 gives a dose titration guideline. The average dose in a Phase 3 trial designed to lower serum phosphorus to 5.0 mg/dL or less was approximately three Renagel 800 mg tablets per meal. The maximum average daily Renagel dose studied was 13 g.
Table 3: Dose Titration Guideline Serum Phosphorus Renagel Dose >5.5 mg/dL Increase 1 tablet per meal at 2-week intervals 3.5–5.5 mg/dL Maintain current dose <3.5 mg/dL Decrease 1 tablet per meal
3 Dosage Forms And Strengths
Tablets: 800 mg white, oval, film-coated, compressed tablets imprinted with RENAGEL 800.
- Tablets: 800 mg (
3 )
4 Contraindications
Renagel is contraindicated in patients with bowel obstruction.
Renagel is contraindicated in patients with known hypersensitivity to sevelamer hydrochloride or to any of the excipients.
- Bowel obstruction. (
4 )- Known hypersensitivity to sevelamer hydrochloride or to any of the excipients. (
4 )
5 Warnings And Precautions
- Serious cases of dysphagia, bowel obstruction, bleeding gastrointestinal ulcers, colitis, ulceration, necrosis, and perforation have been associated with sevelamer use, some requiring hospitalization and surgery. (
5.1 )5.1 Gastrointestinal Adverse Events
Patients with dysphagia, swallowing disorders, severe gastrointestinal (GI) motility disorders, including severe constipation, or major GI tract surgery were not included in the Renagel clinical studies.
Dysphagia and esophageal tablet retention have been reported in association with use of sevelamer tablets, some requiring hospitalization and intervention. Consider using sevelamer suspension in patients with a history of swallowing disorders.
Cases of bowel obstruction, bleeding gastrointestinal ulcers, colitis, ulceration, necrosis, and perforation have also been reported with sevelamer use [see Adverse Reactions (6.2)] . Inflammatory disorders may resolve upon Renagel discontinuation. Treatment with Renagel should be re-evaluated in patients who develop severe gastrointestinal symptoms.
5.2 Monitor Serum Chemistries
Bicarbonate and chloride levels should be monitored.
5.3 Monitor for Reduced Vitamins D, E, K (clotting factors) and Folic Acid Levels
In preclinical studies in rats and dogs, sevelamer hydrochloride reduced vitamins D, E, and K (coagulation parameters) and folic acid levels at doses of 6 to 10 times the recommended human dose. In short-term clinical trials, there was no evidence of reduction in serum levels of vitamins. However, in a one-year clinical trial, 25-hydroxyvitamin D (normal range 10 to 55 ng/mL) fell from 39 ± 22 ng/mL to 34 ± 22 ng/mL (p<0.01) with sevelamer hydrochloride treatment. Most (approximately 75%) patients in sevelamer hydrochloride clinical trials received vitamin supplements, which is typical of patients on dialysis.
6 Adverse Reactions
- The most common reasons for discontinuing treatment were gastrointestinal adverse reactions. (
6.1 )- In a parallel design study of 12 weeks duration, treatment-emergent adverse reactions to Renagel tablets in peritoneal dialysis patients included dyspepsia (12%), peritonitis (8%), diarrhea (5%), nausea (5%), constipation (4%), pruritus (4%), abdominal distension (3%), vomiting (3%), fatigue (3%), anorexia (3%), and arthralgia (3%). (
6.1 )- Cases of fecal impaction and, less commonly, ileus, bowel obstruction, and bowel perforation have been reported. (
6.2 )
To report SUSPECTED ADVERSE REACTIONS, contact Genzyme Corporation at 1-800-847-0069 and or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In a parallel design study of sevelamer hydrochloride with treatment duration of 52 weeks, adverse reactions reported for sevelamer hydrochloride (n=99) were similar to those reported for the active-control group (n=101). Overall adverse reactions among those treated with sevelamer hydrochloride occurring in >5% of patients included: vomiting (22%), nausea (20%), diarrhea (19%), dyspepsia (16%), abdominal pain (9%), flatulence (8%), and constipation (8%). A total of 27 patients treated with sevelamer and 10 patients treated with comparator withdrew from the study due to adverse reactions.
Based on studies of 8 to 52 weeks, the most common reason for withdrawal from Renagel was gastrointestinal adverse reactions (3%–16%).
In 143 peritoneal dialysis patients studied for 12 weeks, most adverse reactions were similar to adverse reactions observed in hemodialysis patients. The most frequently occurring treatment-emergent serious adverse reaction was peritonitis (8 reactions in 8 patients [8%] in the sevelamer group and 2 reactions in 2 patients [4%] on active control). Thirteen patients (14%) in the sevelamer group and 9 patients (20%) in the active-control group discontinued, mostly for gastrointestinal adverse reactions. Patients on peritoneal dialysis should be closely monitored to ensure the reliable use of appropriate aseptic technique with the prompt recognition and management of any signs and symptoms associated with peritonitis.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of sevelamer hydrochloride (Renagel): hypersensitivity, pruritus, rash, abdominal pain, bleeding gastrointestinal ulcers, colitis, ulceration, necrosis, fecal impaction and uncommon cases of ileus, intestinal obstruction, and intestinal perforation. Appropriate medical management should be given to patients who develop constipation or have worsening of existing constipation to avoid severe complications.
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency or to establish a causal relationship to drug exposure.
7 Drug Interactions
There are no empirical data on avoiding drug interactions between Renagel and most concomitant oral drugs. For oral medication where a reduction in the bioavailability of that medication would have a clinically significant effect on its safety or efficacy (e.g., cyclosporine, tacrolimus, levothyroxine), consider separation of the timing of the administration of the two drugs [see Clinical Pharmacology (12.3)] . The duration of separation depends upon the absorption characteristics of the medication concomitantly administered, such as the time to reach peak systemic levels and whether the drug is an immediate-release or an extended-release product. Where possible monitor clinical responses or blood levels of concomitant drugs that have a narrow therapeutic range.
Table 4: Sevelamer Drug Interactions Oral drugs for which sevelamer did not alter the pharmacokinetics when administered concomitantly Digoxin Enalapril Iron Metoprolol Warfarin Oral drugs that have demonstrated interaction with sevelamer and are to be dosed separately from Renagel Dosing Recommendations Ciprofloxacin Take at least 2 hours before or 6 hours after sevelamer Mycophenolate mofetil Take at least 2 hours before sevelamer
- When clinically significant drug interactions are expected, separate the timing of administration and monitor clinical responses or blood levels of the concomitant medication. (
7 )- Sevelamer did not alter the pharmacokinetics of digoxin, enalapril, iron, metoprolol, and warfarin. (
7 )- Sevelamer binds ciprofloxacin and mycophenolate mofetil; dose these drugs separately from Renagel. (
7 )
8 Use In Specific Populations
8.1 Pregnancy
Risk Summary
Sevelamer hydrochloride is not absorbed systemically following oral administration and maternal use is not expected to result in fetal exposure to the drug.
Clinical Considerations
Sevelamer hydrochloride may decrease serum levels of fat-soluble vitamins and folic acid in pregnant women [see Clinical Pharmacology (12.2)] . Consider supplementing with these vitamins.
Data
Animal data
In pregnant rats given dietary doses of 0.5, 1.5, or 4.5 g/kg/day of Renagel during organogenesis, reduced or irregular ossification of fetal bones, probably due to a reduced absorption of fat-soluble vitamin D, occurred at 7–21 times the maximum human equivalent dose of 13 g based on 60 kg body weight. In pregnant rabbits given oral doses of 100, 500, or 1000 mg/kg/day of Renagel by gavage during organogenesis, an increase of early resorptions occurred in the high-dose group (human equivalent dose approximately 5 times the maximum clinical trial dose based on 60 kg body weight).
8.2 Lactation
Risk Summary
Renagel is not absorbed systemically by the mother following oral administration and breastfeeding is not expected to result in exposure of the child to Renagel.
Clinical Considerations
Sevelamer hydrochloride may decrease serum levels of fat-soluble vitamins and folic acid in lactating women [see Clinical Pharmacology (12.2)] . Consider supplementing with these vitamins.
8.4 Pediatric Use
The safety and efficacy of Renagel has not been established in pediatric patients.
8.5 Geriatric Use
Clinical studies of Renagel did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range.
10 Overdosage
Renagel has been given to normal healthy volunteers in doses of up to 14 g per day for eight days with no adverse effects. Renagel has been given in average doses up to 13 g per day to hemodialysis patients. There are no reports of overdosage with Renagel in patients. Since Renagel is not absorbed, the risk of systemic toxicity is low.
11 Description
The active ingredient in Renagel tablets is sevelamer hydrochloride, a polymeric amine that binds phosphate and is meant for oral administration. Sevelamer hydrochloride is poly(allylamine hydrochloride) crosslinked with epichlorohydrin in which 40% of the amines are protonated. It is known chemically as poly(allylamine- co-N,N'-diallyl-1,3-diamino-2-hydroxypropane) hydrochloride. Sevelamer hydrochloride is hydrophilic, but insoluble in water. The structure is represented in Figure 1.
Figure 1: Chemical Structure of Sevelamer Hydrochloride
![]()
a, b = number of primary amine groups a + b = 9 c = number of crosslinking groups c = 1 n = fraction of protonated amines n = 0.4 m = large number to indicate extended polymer network
The primary amine groups shown in the structure are derived directly from poly(allylamine hydrochloride). The crosslinking groups consist of two secondary amine groups derived from poly(allylamine hydrochloride) and one molecule of epichlorohydrin.
Renagel tablets: Each film-coated tablet of Renagel contains 800 mg of sevelamer hydrochloride on an anhydrous basis. The inactive ingredients are hypromellose, diacetylated monoglyceride, colloidal silicon dioxide, and stearic acid. The tablet imprint contains iron oxide black ink.
12 Clinical Pharmacology
12.1 Mechanism of Action
Renagel contains sevelamer hydrochloride, a non-absorbed binding crosslinked polymer. It contains multiple amines separated by one carbon from the polymer backbone. These amines exist in a protonated form in the intestine and interact with phosphate molecules through ionic and hydrogen bonding. By binding phosphate in the dietary tract and decreasing absorption, sevelamer hydrochloride lowers the phosphate concentration in the serum.
12.2 Pharmacodynamics
In addition to effects on serum phosphate levels, sevelamer hydrochloride has been shown to bind bile acids in vitroand in vivoin experimental animal models. Bile acid binding by ion exchange resins is a well-established method of lowering blood cholesterol. Because sevelamer binds bile acids, it may interfere with normal fat absorption and thus may reduce absorption of fat-soluble vitamins such as A, D, and K. In clinical trials of sevelamer hydrochloride, both the mean total and LDL cholesterol declined by 15% to 31%. This effect is observed after 2 weeks. Triglycerides, HDL cholesterol, and albumin did not change.
12.3 Pharmacokinetics
A mass balance study using 14C-sevelamer hydrochloride in 16 healthy male and female volunteers showed that sevelamer hydrochloride is not systemically absorbed. No absorption studies have been performed in patients with renal disease.
Drug Interactions
In vivo
Sevelamer carbonate has been studied in human drug-drug interaction studies (9.6 g once daily with a meal) with warfarin and digoxin. Sevelamer hydrochloride, which contains the same active moiety as sevelamer carbonate, has been studied in human drug-drug interaction studies (2.4–2.8 g single dose or three times daily with meals or two times daily without meals) with ciprofloxacin, digoxin, enalapril, iron, metoprolol, mycophenolate mofetil and warfarin.
Coadministered single dose of 2.8 g of sevelamer hydrochloride in fasted state decreased the bioavailability of ciprofloxacin by approximately 50% in healthy subjects.
Concomitant administration of sevelamer and mycophenolate mofetil in adult and pediatric patients decreased the mean MPA C maxand AUC 0–12hby 36% and 26%, respectively.
Sevelamer carbonate or sevelamer hydrochloride did not alter the pharmacokinetics of a single dose of enalapril, digoxin, iron, metoprolol and warfarin when coadministered.
During postmarketing experience, cases of increased thyroid stimulating hormone (TSH) levels have been reported in patients coadministered sevelamer hydrochloride and levothyroxine. Reduction in concentrations of cyclosporine and tacrolimus leading to dose increases has also been reported in transplant patients when coadministered with sevelamer hydrochloride without any clinical consequences (e.g., graft rejection). The possibility of an interaction cannot be excluded with these drugs.
13 Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Standard lifetime carcinogenicity bioassays were conducted in mice and rats. Rats were given sevelamer hydrochloride by diet at 0.3, 1, or 3 g/kg/day. There was an increased incidence of urinary bladder transitional cell papilloma in male rats of the high-dose group (human equivalent dose twice the maximum clinical trial dose of 13 g). Mice received dietary administration of sevelamer hydrochloride at doses of up to 9 g/kg/day (human equivalent dose 3 times the maximum clinical trial dose). There was no increased incidence of tumors observed in mice.
In an in vitromammalian cytogenetic test with metabolic activation, sevelamer hydrochloride caused a statistically significant increase in the number of structural chromosome aberrations. Sevelamer hydrochloride was not mutagenic in the Ames bacterial mutation assay.
Sevelamer hydrochloride did not impair the fertility of male or female rats in a dietary administration study in which the females were treated from 14 days prior to mating through gestation and the males were treated for 28 days prior to mating. The highest dose in this study was 4.5 g/kg/day (human equivalent dose 3 times the maximum clinical trial dose of 13 g).
14 Clinical Studies
The ability of Renagel to lower serum phosphorus in CKD patients on dialysis was demonstrated in six clinical trials: one double-blind placebo-controlled 2-week study (Renagel N=24); two open-label uncontrolled 8-week studies (Renagel N=220) and three active-controlled open-label studies with treatment durations of 8 to 52 weeks (Renagel N=256). Three of the active-controlled studies are described here. One is a crossover study with two 8-week periods comparing Renagel to an active control. The second is a 52-week parallel study comparing Renagel with active control. The third is a 12-week parallel study comparing Renagel and active control in peritoneal dialysis patients.
14.1 Active-Control, Cross-Over Study in Hemodialysis Patients
Eighty-four CKD patients on hemodialysis who were hyperphosphatemic (serum phosphorus >6.0 mg/dL) following a two-week phosphate-binder washout period received Renagel and active control for eight weeks each in random order. Treatment periods were separated by a two-week phosphate-binder washout period. Patients started on treatment three times per day with meals. Over each eight-week treatment period, at three separate time points the dose of Renagel could be titrated up 1 capsule or tablet per meal (3 per day) to control serum phosphorus, the dose of active control could also be altered to attain phosphate control. Both treatments significantly decreased mean serum phosphorus by about 2 mg/dL (Table 5).
Table 5: Mean Serum Phosphorus (mg/dL) at Baseline and Endpoint Renagel (N=81) Active-Control (N=83) Baseline at End of Washout 8.4 8.0 Endpoint 6.4 5.9 Change from Baseline at Endpoint (95% Confidence Interval) -2.0 p<0.0001, within treatment group comparison (-2.5, -1.5)-2.1 (-2.6, -1.7)
The distribution of responses is shown in Figure 2. The distributions are similar for sevelamer hydrochloride and active control. The median response is a reduction of about 2 mg/dL in both groups. About 50% of subjects have reductions between 1 and 3 mg/dL.
Figure 2: Percentage of Patients (Y-axis) Attaining a Phosphorus Reduction from Baseline (mg/dL) at Least as Great as the Value of the X-axis
![]()
Average daily Renagel dose at the end of treatment was 4.9 g (range of 0.0 to 12.6 g).
14.2 Active-Control, Parallel Study in Hemodialysis Patients
Two hundred CKD patients on hemodialysis who were hyperphosphatemic (serum phosphorus >5.5 mg/dL) following a two-week phosphate-binder washout period were randomized to receive Renagel 800 mg tablets (N=99) or an active control (N=101). The two treatments produced similar decreases in serum phosphorus. At week 52, using last observation carried forward, Renagel and active control both significantly decreased mean serum phosphorus (Table 6).
Table 6: Mean Serum Phosphorus (mg/dL) and Ion Product at Baseline and Change from Baseline to End of Treatment Renagel (N=94) Active-Control (N=98) Phosphorus Baseline 7.5 7.3 Change from Baseline at Endpoint -2.1 -1.8 Ca × Phosphorus Ion Product Baseline 70.5 68.4 Change from Baseline at Endpoint -19.4 -14.2
Sixty-one percent of Renagel patients and 73% of the control patients completed the full 52 weeks of treatment.
Figure 3, a plot of the phosphorus change from baseline for the completers, illustrates the durability of response for patients who are able to remain on treatment.
Figure 3: Mean Phosphorus Change from Baseline for Patients who Completed 52 Weeks of Treatment
![]()
Average daily Renagel dose at the end of treatment was 6.5 g (range of 0.8 to 13 g).
14.3 Active-Control, Parallel Study in Peritoneal Dialysis Patients
One hundred and forty-three patients on peritoneal dialysis, who were hyperphosphatemic (serum phosphorus >5.5 mg/dL) following a two-week phosphate-binder washout period, were randomized to receive Renagel (N=97) or active control (N=46) open label for 12 weeks. Average daily Renagel dose at the end of treatment was 5.9 g (range 0.8 to 14.3 g). There were statistically significant changes in serum phosphorus (p<0.001) for Renagel (-1.6 mg/dL from baseline of 7.5 mg/dL), similar to the active control.
16 How Supplied/storage And Handling
Renagel ®(sevelamer hydrochloride) tablets are supplied as white, oval, film-coated, compressed tablets, imprinted with RENAGEL 800 containing 800 mg of sevelamer hydrochloride. Renagel 800 mg Tablets are packaged in bottles of 180 tablets.
1 Bottle of 180 ct 800 mg Tablets (NDC 58468-0021-1)
STORAGE AND HANDLING SECTION
Storage:Store at 25°C (77°F): excursions permitted to 15°C–30°C (59°F–86°F).
Do not use Renagel after the expiration date on the bottle.
[See USP controlled room temperature]
Protect from moisture.
17 Patient Counseling Information
Advise patients to take Renagel with meals and adhere to their prescribed diets.
Provide instructions on concomitant medications that should be dosed apart from Renagel.
Advise patients to report new onset or worsening of existing constipation or bloody stools promptly to their healthcare provider [see Warnings and Precautions (5.1)] .
Manufactured by: Genzyme Ireland Ltd.
For: Genzyme Corporation Cambridge, MA 02141 A SANOFI COMPANY
Renagel is a Registered Trademark of Genzyme Corporation.
Package Label.principal Display Panel
Bottle Label - Principal Display Panel – 400 mg - U.K. Source
EACH TABLET CONTAINS:
Active Ingredient: Sevelamer hydrochloride….400 mg.
Inactive Ingredients:
Hypromellose, diacetylated monoglyceride, colloidal silicon dioxide, and stearic acid.
Dispense in a tight container.
Protect from moisture.
Store at 25ºC (77ºF).
GENZYME
NDC 58468-0020-1
Renagel ®Tablets
(sevelamer hydrochloride) 400 mg
360 Tablets
Rx only
Manufactured by:
Genzyme Ireland Ltd.
For: Genzyme Corporation
500 Kendall Street
Cambridge, MA 02142 USA
Country of origin: U.K.
400 mg
USUAL DOSAGE:
SEE PACKAGE INSERT FOR DOSAGE INFORMATION.
![]()
Principal Display Panel - 800 Mg Tablet Bottle Label
NDC 58468- 0021-1 Rx only
Renagel ®Tablets (sevelamer hydrochloride) 800 mg
180 TABLETS
Manufactured By: Genzyme Ireland Ltd. For: Genzyme Corporation Cambridge, MA 02142
Origin U.K.
800 mg
![]()
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site