Sodium Sulfacetamide Dailymed
Generic: sodium sulfacetamide is used for the treatment of Corneal Ulcer Ear Diseases Infant, Newborn Dermatitis, Seborrheic Trachoma Skin Diseases, Bacterial
Go PRO for all pill images
Inactive Ingredients
Butylated Hydroxytoluene, Citric Acid, Cetyl Alcohol, Cocamidopropyl Betaine, Disodium EDTA, Glycerin, Glyceryl Stearate SE, PEG-100 Stearate, Phenoxyethanol, Purified Water, Sodium Laureth Sulfate, Sodium Thiosulfate, Stearyl Alcohol, Triacetin, Xanthan Gum.
Description
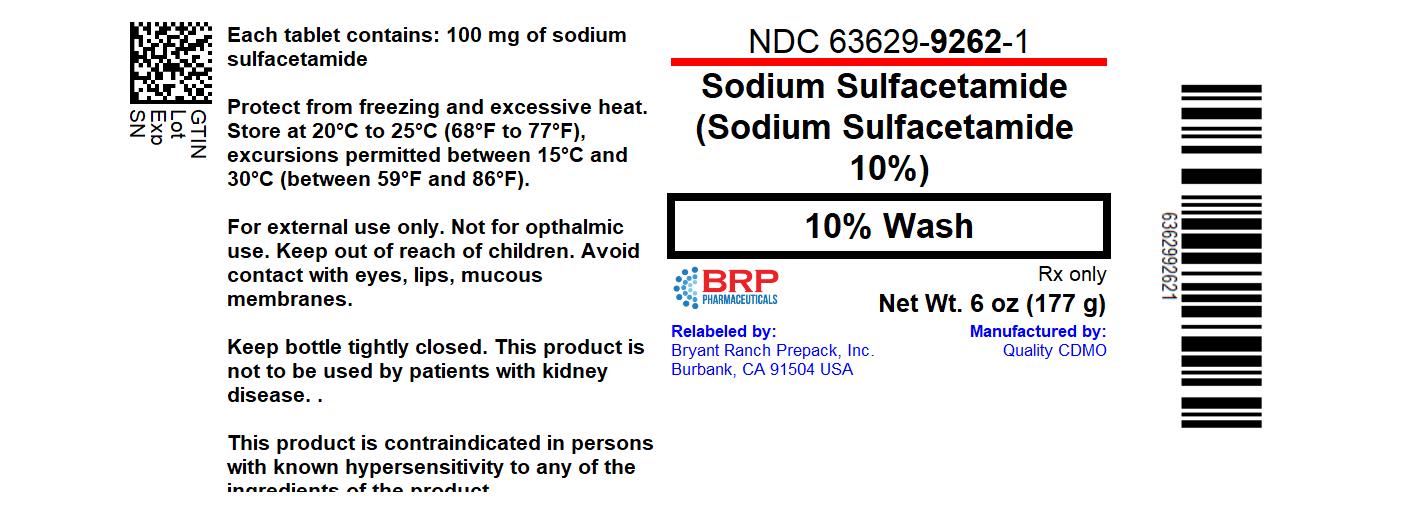
Each gram contains 100 mg of sodium sulfacetamide in a vehicle consisting of: Butylated Hydroxytoluene, Citric Acid, Cetyl Alcohol, Cocamidopropyl Betaine, Disodium EDTA, Glycerin, Glyceryl Stearate SE, PEG-100 Stearate, Phenoxyethanol, Purified Water, Sodium Laureth Sulfate, Sodium Thiosulfate, Stearyl Alcohol, Triacetin, Xanthan Gum.
Indications
This product is intended for topical application in the following scaling dermatoses: seborrheic dermatitis and seborrhea sicca (dandruff). It also is indicated for the treatment of secondary bacterial infections of the skin due to organisms susceptible to sulfonamides.
Contraindications
This product is contraindicated in persons with known or suspected hypersensitivity to any of the ingredients of the product. This product is not to be used by patients with kidney disease.
Dosage And Administration
Seborrheic dermatitis including seborrhea sicca - Wash affected areas twice daily (morning and evening) or as directed by your physician. Avoid contact with eyes or mucous membranes. Wet skin and liberally apply to areas to be cleansed, massage gently into skin working into a full lather, rinse thoroughly, pat dry and repeat after 10 to 20 seconds. Rinsing with plain water will remove any excess medication. Repeat application as described above for 8 to 10 days or as directed by your physician. lf skin dryness occurs it may be controlled by rinsing cleanser off sooner or using less frequently. Regular shampooing following the use of this product is not necessary, but the hair should be shampooed at least once a week. As the condition subsides, the interval between applications may be lengthened. Applications once or twice weekly or every other week may prevent recurrence. Should the condition recur after stopping therapy, the application of this product should be reinitiated as at the beginning of treatment.
Secondary cutaneous bacterial infections - Wash affected areas twice daily (morning and evening) or as directed by your physician. Avoid contact with eyes or mucous membranes. Wet skin and liberally apply to areas to be cleansed, massage gently into skin for 10 to 20 seconds working into a full lather, rinse thoroughly and pat dry. Rinsing with plain water will remove any excess medication. Repeat application as described above for 8 to 10 days or as directed by your physician. If skin dryness occurs it may be controlled by rinsing cleanser off sooner or using less frequently. See package insert for full prescribing information.
Warnings
WARNING: FOR EXTERNAL USE ONLY. NOT FOR OPHTHALMIC USE. KEEP OUT OF REACH OF CHILDREN.
Avoid contact with eyes, lips and mucous membranes.
See label booklet for Full prescribing information.
Storage
Store at 20°C to 25°C (68°F to 77°F), excursions permitted between 15°C and 30°C (between 59°F and 86°F). Protect from freezing and excessive heat. Keep bottle tightly closed.
This bottle is not filled to the top but does contain 6 fl oz of product as identified on the front panel of the bottle.
To report a serious adverse event or obtain product information, call (877) 250-3427.
How Supplied
Sodium Sulfacetamide 10% Wash
- NDC 63629-9262-1: 177 g in a BOTTLE
Repackaged/Relabeled by:Bryant Ranch Prepack, Inc.Burbank, CA 91504
Package Label.principal Display Panel
Sodium Sulfacetamide 10% Wash
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site

