Chemet (succimer 100 mg) Dailymed
Generic: succimer is used for the treatment of Lead Poisoning, Nervous System, Childhood
Go PRO for all pill images
1 Indications And Usage
CHEMET is indicated for the treatment of lead poisoning in pediatric patients aged 1 year and older with blood lead levels above 45 mcg/dL.
CHEMET is a lead chelator indicated for the treatment of lead poisoning in pediatric patients aged 1 year and older with blood lead levels above 45 mcg/dL. (1 )
Limitations of Use
- CHEMET is not indicated for prophylaxis of lead poisoning in a lead-containing environment. (
1 )- CHEMET does not cross the blood-brain barrier and is not indicated to treat encephalopathy associated with lead toxicity. (
1 )
Limitations of Use
- CHEMET is not indicated for prophylaxis of lead poisoning in a lead-containing environment.
- CHEMET does not cross the blood-brain barrier and is not indicated to treat encephalopathy associated with lead toxicity.
2 Dosage And Administration
- See Full Prescribing Information for important pretreatment evaluations. (
2.1 )- Ensure patients receiving CHEMET are adequately hydrated. (
2.3 )- Administer CHEMET capsules whole when possible. (
2.3 )- Pediatric patients who cannot swallow whole capsules: Sprinkle contents of capsule in food (or on a spoon followed by a drink). (
2.3 )- Recommended Dosage: 10 mg/kg or 350 mg/m2 orally every 8 hours for five days followed by 10 mg/kg or 350 mg/m2 orally every 12 hours for an additional 14 days. (
2.2 )2.1 Important Pretreatment Evaluations
- Identify the source of lead in the pediatric patient's environment and eliminate the source prior to beginning treatment with CHEMET.
- Assess the following before initiating treatment with CHEMET:
- Blood lead concentration
- Complete blood count (CBC) with differential and platelets [see Dosage and Administration (2.2), Warnings and Precautions (5.2)]
- Ensure absolute neutrophil count (ANC) > 1500/mcL [see Dosage and Administration (2.2)]
- Transaminases (AST/ALT) [see Warnings and Precautions (5.3) and Use in Specific Populations (8.6)]
- Renal function with blood urea nitrogen (BUN), creatinine, urinary protein [see Use in Specific Populations (8.5)].
- Patients who have previously received Edetate calcium disodium (CaNa2EDTA) with or without dimercaprol may receive CHEMET for subsequent treatment after an interval of four weeks.
2.2 Recommended Dosage
The recommended dosage of CHEMET for pediatric patients with lead poisoning is 10 mg/kg or 350 mg/m2 orally every 8 hours for five days followed by 10 mg/kg or 350 mg/m2 orally every 12 hours for an additional 14 days [see Table 1 CHEMET Pediatric Dosing Chart]. Initiation of therapy at higher doses is not recommended. The total treatment course consists of 19 days.
After discontinuation of CHEMET, elevated blood levels and associated symptoms may return rapidly because of redistribution of lead from bone stores to soft tissues and blood. Assess blood lead concentration after the completion of a 19 day course and every week until stable. Repeated courses may be administered after two weeks off treatment if blood lead concentrations remain elevated. A minimum of two weeks between treatment courses is recommended unless blood lead concentrations indicate the need for more prompt treatment.
Table 1. CHEMET Pediatric Dosing Chart Weight in Kilograms (kg) Dose (mg) Number of Capsules 8 to15 kg 100 mg 1 16 to 23 kg 200 mg 2 24 to 34 kg 300 mg 3 35 to 44 kg 400 mg 4 >45 kg 500 mg 5
The safety of uninterrupted dosing longer than 3 weeks has not been established and is not recommended.
Dosage Modifications for Neutropenia
Monitor CBCs weekly.
If the absolute neutrophil count (ANC) is <1200/mcL interrupt CHEMET.
- Resume CHEMET when ANC has recovered to >1500/mcL (or the patient's baseline count).
- Immediately discontinue CHEMET for signs/symptoms of infection.
- Only rechallenge patients who developed neutropenia with CHEMET therapy if the benefit clearly outweighs the potential risk.
2.3 Preparation and Administration Instructions
Administer CHEMET capsules whole.
In pediatric patients who cannot swallow the capsules whole, separate the capsule and sprinkle the medicated beads on a small amount of soft food or put them in a spoon and follow with a fruit drink.
Ensure that all patients receiving CHEMET are adequately hydrated [see Use in Specific Populations (8.5), Pharmacokinetics (12.3)].
3 Dosage Forms And Strengths
Capsules: 100 mg, opaque white, imprinted black with "CHEMET 100".
Capsule: 100 mg (3 )
4 Contraindications
CHEMET is contraindicated in patients with a history of hypersensitivity reaction to succimer. Reactions have included mucocutaneous vesicular eruptions, urticaria, and angioedema [see Warnings and Precautions (5.1)].
Patients with a history of hypersensitivity reaction to succimer. (4 )
5 Warnings And Precautions
- Hypersensitivity and dermatologic reactions: Interrupt treatment if rash or mucocutaneous vesicular eruptions occur. (
5.1 )- Neutropenia: Monitor complete blood counts, interrupt treatment for ANC below 1200/mcL, and monitor for infection. (
5.2 )- Hepatic Toxicity: Monitor hepatic transaminases (ALT/AST); interrupt treatment if above 5 times ULN. (
5.3 )- Embryo-Fetal Toxicity: May cause fetal harm when administered to a pregnant woman. (
5.5 )5.1 Hypersensitivity and Dermatologic Reactions
CHEMET can cause hypersensitivity reactions and dermatologic reactions.
Rash
Rash occurs in approximately 4% of patients treated with CHEMET. Interrupt treatment if rash occurs. Consider rechallenge if lead levels are high enough to warrant retreatment.
Hypersensitivity reactions including urticaria and angioedema have been reported on repeated administration of CHEMET [see Contraindications (4)].
Mucocutaneous Reactions
Mucocutaneous vesicular eruptions can occur with CHEMET use and may increase with each treatment course. Monitor patients requiring repeated CHEMET courses for the occurrence of mucocutaneous eruptions, including oral, urethral, and perianal. Interrupt treatment if mucocutaneous vesicular eruptions occur.
5.2 Neutropenia
Iron chelators, including CHEMET, can cause neutropenia. Monitoring of complete blood counts is recommended [see Dosage and Administration (2.2)]. Interrupt treatment if absolute neutrophil count (ANC) is <1200/mcL and interrupt treatment until recovery to above 1500/mcL (or the patient's baseline count). Only rechallenge patients who developed neutropenia with CHEMET therapy if the benefit clearly outweighs the potential risk. If rechallenge is attempted, monitor CBC more frequently.
Monitor for signs and symptoms of infection and immediately discontinue CHEMET if they develop.
5.3 Hepatic Toxicity
Elevated transaminases (ALT/AST) occurred in 6-10% of patients treated with CHEMET. Monitor serum AST and ALT at baseline and at least weekly during treatment. Monitor patients with a history of liver disease more frequently. Serum aminotransferase elevations above 5 times the upper limit of normal (if confirmed) should lead to dose reduction or temporary cessation.
5.4 Embryo-Fetal Toxicity
Based on findings from animal reproduction studies, CHEMET may cause fetal harm when administered to a pregnant woman. Advise females of reproductive potential to use an effective method of contraception during treatment with CHEMET and for 14 days after the final dose [see Use in Specific Populations (8.1, 8.3)].
5.5 Laboratory Test Interference
CHEMET may interfere with serum and urinary laboratory tests [see Drug Interactions (7.1)].
6 Adverse Reactions
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Hypersensitivity and Dermatologic Reactions [see Warnings and Precautions (5.1)]
- Neutropenia [see Warnings and Precautions (5.2)]
- Hepatic Toxicity [see Warnings and Precautions (5.3)]
Most common adverse reactions (incidence ≥ 10%) in
- Pediatric patients: Digestive (nausea, vomiting, diarrhea, appetite loss, hemorrhoidal symptoms, metallic taste in mouth). (
6.1 )
To report SUSPECTED ADVERSE REACTIONS, contact Recordati Rare Diseases Inc. at 1-888-575-8344 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Table 2 presents the adverse reactions associated with Chemet in pediatric patients.
Table 2 Incidence of Adverse Reactions Associated with Chemet in Pediatric Patients Body System: Adverse Reactions Pediatric Patients(n=191) Digestive Nausea, vomiting, diarrhea, appetite loss, hemorrhoidal symptoms, metallic taste in mouth 12% Body as a Whole Back pain, abdominal cramps, stomach pains, head pain, rib pain, chills, flank pain, fever, flu-like symptoms, heavy head/tired, head cold, headache, moniliasis. 5% Metabolic Elevated ALT or AST, alkaline phosphatase, serum cholesterol. 4% Respiratory Sore throat, rhinorrhea, nasal congestion, cough. 4% Skin Papular rash, herpetic rash, rash, mucocutaneous eruptions, pruritis. 3% Nervous Drowsiness, dizziness, sensorimotor neuropathy, sleepiness, paresthesia. 1% Special Senses Cloudy film in eye, ears plugged, otitis media, eyes watery. 1% Heme/Lymphatic Neutropenia, increased platelet count, eosinophilia 1%
7 Drug Interactions
CHEMET may interfere with serum and urinary laboratory test. (7.1 )
7.1 Laboratory Test Interference
CHEMET may interfere with serum and urinary laboratory tests. In vitro studies have shown CHEMET to cause false positive results for ketones in urine using nitroprusside reagents and falsely decreased measurements of serum uric acid and creatinine phosphokinase (CPK).
7.2 Use with Other Chelation Therapies
Concomitant administration of CHEMET with other chelation therapy, such as CaNa2EDTA is not recommended.
8 Use In Specific Populations
Pregnancy: May cause fetal harm. (8.1 )
Renal Impairment: Assess renal function. (8.6 )
Hepatic Impairment: Assess hepatic function. (8.7)
8.1 Pregnancy
Risk Summary
There are no studies with the use of CHEMET in pregnant women to inform drug-associated risks.
Administration of CHEMET to pregnant mice during organogenesis at dose exposure of 11-times the human exposure at the maximum recommended human dose (MRHD) of 700 mg based on body surface area (BSA) resulted in maternal toxicity and mortality and impaired reflex development in offspring (see Animal Data). There are adverse effects on maternal and fetal outcomes associated with lead poisoning in pregnancy (see Clinical Considerations). CHEMET should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population(s) is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. However, the background risk in the U.S general population of major birth defects is 2 to 4% and of miscarriage is 15 to 20% of clinically recognized pregnancies.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
Lead exposure in pregnancy may increase the risk of gestational hypertension.
Lead crosses the placenta in amounts related to maternal plasma levels. Prenatal lead exposure may be associated with spontaneous abortion, preterm delivery, decreased birth weight, and impaired neurodevelopment.
Data
Animal Data
In embryo-fetal developmental studies, pregnant mice received subcutaneous succimer during the period of organogenesis at doses up to 1640 mg/kg/day (11-times the MRHD based on BSA) which resulted in both maternal and fetal toxicity.
In a developmental study in rats, dosing with succimer during the period of organogenesis resulted in maternal toxicity and deaths at the dose of 720 mg/kg/day (10-times the MRHD based on BSA) or more. The dose of 510 mg/kg/day (7-times the MRHD based on BSA) was the highest tolerable dose in pregnant rats. Impaired development of reflexes was noted in pups of dams receiving 720 mg/kg/day (10-times the MRHD based on BSA).
8.2 Lactation
Risk Summary
There are no data on the presence of succimer or its metabolite in either human or animal milk, the effects on the breastfed infant, or the effects on milk production. There are clinical considerations regarding lead in breastmilk (see Clinical Considerations).
Clinical Considerations
When used for the treatment of lead poisoning, the amount of lead in breast milk may range from 0.6% to 3% of the maternal serum concentration. Females with confirmed blood lead levels ≥40 mcg/dL should not initiate breastfeeding; pumping and discarding breast milk is recommended until blood lead levels are <40 mcg/dL, at which point breastfeeding may resume. Calcium supplementation may reduce the amount of lead in breast milk.
8.3 Females and Males of Reproductive Potential
CHEMET may cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
Pregnancy Testing
Pregnancy testing is recommended for females of reproductive potential prior to treatment with CHEMET.
Contraception
Females
Advise females of reproductive potential to use effective contraception during treatment with CHEMET and for 14 days after the final dose.
8.4 Pediatric Use
The safety and effectiveness of CHEMET for the treatment of lead poisoning in patients with blood levels above 45 mcg/mL have been established in pediatric patients aged 1 year and older. The safety and effectiveness of CHEMET have not been established in pediatric patients younger than 1 year of age.
8.5 Renal Impairment
Assess renal function prior to and periodically during prolonged therapy. Adequately hydrate patients during therapy. Limited data suggests that succimer is dialyzable, but the lead chelates are not. Monitor patients with a history of renal impairment more frequently.
8.6 Hepatic Impairment
Assess hepatic function prior to and periodically during therapy. Monitor patients with a history of liver disease more frequently [see Warnings and Precautions (5.3)].
10 Overdosage
Doses of 2300 mg/kg in the rat and 2400 mg/kg in the mouse produced ataxia, convulsions, labored respiration and frequently death. Limited data indicate that CHEMET is dialyzable. In case of acute overdosage, consider use of induction of vomiting or gastric lavage followed by administration of an activated charcoal slurry and appropriate supportive therapy.
11 Description
CHEMET (succimer) is an orally active, lead chelating agent. The chemical name for succimer is meso2, 3-dimercaptosuccinic acid (DMSA). Its empirical formula is C4H6O4S2 and molecular weight is 182.2. The meso-structural formula is:
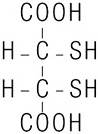
Succimer is a white crystalline powder with an unpleasant, characteristic mercaptan odor and taste.
Each CHEMET opaque white capsule for oral administration contains medicated beads with 100 mg of succimer and the following inactive ingredients: povidone, sodium starch glycolate, and sugar spheres. The capsule shell contains benzyl alcohol, butylparaben, edetate calcium disodium, gelatin, methylparaben, propylparaben, sodium lauryl sulfate, sodium propionate, titanium dioxide, and is imprinted with edible black ink.
12 Clinical Pharmacology
12.1 Mechanism of Action
Succimer is a lead chelator; it forms water soluble chelates and, consequently, increases the urinary excretion of lead.
12.2 Pharmacodynamics
Effect on Essential Minerals
In dose ranging studies performed in 18 men with blood lead levels of 44-96 mcg/dL, three groups of 6 patients received either 10, 6.7 or 3.3 mg/kg succimer orally every 8 hours for 5 days. After five days the mean blood lead levels of the three groups decreased 72.5%, 58.3% and 35.5% respectively. The mean urinary lead excretions in the initial 24 hours were 28.6, 18.6 and 12.3 times the pretreatment 24 hour urinary lead excretion. CHEMET had no significant effect on the urinary elimination of iron, calcium or magnesium. Zinc excretion doubled during treatment.
12.3 Pharmacokinetics
Absorption
Succimer achieved peak plasma concentrations at 1–2 hours.
Elimination
Metabolism
Approximately 90% of absorbed dose is metabolized to mixed succimer-cysteine disulfides. The majority of mixed disulfides consisted of succimer in disulfide linkages with two molecules of L-cysteine, the remaining disulfides contained one L-cysteine per succimer molecule.
Excretion
In a study performed in healthy adult volunteers, after a single dose of 14C-succimer at 16, 32, or 48 mg/kg, 49% of the radiolabeled dose was excreted on average: 39% in the feces, 9% in the urine and 1% as carbon dioxide from the lungs. The apparent elimination half-life of the radiolabeled material in the blood was about two days.
In other studies of healthy adult volunteers receiving a single oral dose of 10 mg/kg, approximately 25% of the administered dose was excreted in the urine with the peak blood level and urinary excretion occurring between two and four hours. Of the total amount of drug eliminated in the urine, approximately 90% was eliminated in altered form as mixed succimer-cysteine disulfides; the remaining 10% was eliminated unchanged.
13 Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity studies have been conducted with CHEMET. Succimer was not mutagenic in the Ames bacterial reverse mutation assay and in the mammalian cell forward gene mutation assay. Succimer did not show any adverse effects on fertility and reproductive performance in rats up to doses of 510 mg/kg/day in males and 100 mg/kg/day in females (7- and 11-times the MRHD based on BSA, respectively).
14 Clinical Studies
The efficacy of CHEMET in the treatment of lead poisoning in pediatric patients was established in a dose-ranging, actively controlled study of 15 pediatric patients aged 2 to 7 years with blood lead levels of 30-49 mcg/dL and positive CaNa2EDTA lead mobilization tests. Fifteen patients were assigned to a dose of 350 mg/m2 or 233 mg/m2, or 116 mg/m2 (5 patients per group) orally every 8 hours for 5 days. Six control patients received 1000 mg/m2/day CaNa2EDTA intravenously for 5 days. Following therapy, the mean blood lead levels decreased 78, 63, and 42% respectively in the three CHEMET treatment groups. The response of the 350 mg/m2 every 8 hours (10 mg/kg every 8 hours) group was significantly better than that of the other CHEMET dose level groups as well as that of the control group, whose mean blood lead level fell 48%. Although other dosing regimens were used in the study described, only 10 mg/kg or 350 mg/m2 orally every 8 hours for five days followed by 10 mg/kg or 350 mg/m2 orally every 12 hours for an additional 14 days is the recommended dosage.
Patients experienced a rebound in blood lead levels after discontinuation of CHEMET. In these studies, after treatment with a dose of 350 mg/m2 (10 mg/kg) every 8 hours for five days, the mean lead level rebounded and plateaued at 60-85% of pretreatment levels two weeks after therapy.
In an attempt to control rebound of blood lead levels, 19 pediatric patients, ages 1 to 7 years, with blood lead levels of 42-67 mcg/dL, were treated with 350 mg/m2 CHEMET every 8 hours for five days and then divided into three groups. One group was followed for two weeks with no further therapy, the second group was treated for two weeks with 350 mg/m2 daily, and the third with 350 mg/m2 every 12 hours. After the initial 5 days of therapy, the mean blood lead level in all subjects declined 61%. While the untreated group and the group treated with 350 mg/m2 daily experienced rebound during the ensuing two weeks, the group who received the 350 mg/m2 every 12 hours experienced no such rebound during the treatment period and less rebound following cessation of therapy.
In another study, ten pediatric patients, ages 21 to 72 months, with blood lead levels of 30-57 mcg/dL were treated with CHEMET 350 mg/m2 every eight hours for five days followed by an additional 19-22 days of therapy at a dose of 350 mg/m2 every 12 hours. The mean blood lead levels decreased and remained stable at under 15 mcg/dL during the extended dosing period.
In addition to the controlled studies, approximately 250 patients with lead poisoning have been treated with CHEMET either orally or parenterally in open U.S. and foreign studies.
16 How Supplied/storage And Handling
Capsules: 100 mg of CHEMET (succimer), imprinted black with "CHEMET 100", in a bottle of 100 (NDC 55292-201-11).
STORAGE AND HANDLING SECTION
Store between 15°C and 25°C and avoid excessive heat.
Dispense in tight, light-resistant container.
17 Patient Counseling Information
- Advise caregivers and/or patients to maintain adequate fluid intake [see Dosage and Administration (2.3)].
- Advise caregivers and/or patients to contact their healthcare provider should a rash develop [see Warnings and Precautions (5.1)].
- Advise caregivers and/or patients to contact their healthcare provider right away if signs/symptoms of infection develop [see Warnings and Precautions (5.2)].
- Advise caregivers that in pediatric patients who cannot swallow the capsules whole, separate the capsule and sprinkle the medicated beads on a small amount of soft food or put them in a spoon and follow with a fruit drink [see Dosage and Administration (2.3)].
Manufactured for:Recordati Rare Diseases Inc.Bridgewater, NJ 08807
Principal Display Panel - 100 Mg Capsule Bottle Label
NDC 55292-201-11100 Capsules
Chemet® (succimer) Capsules
100 mg
RECORDATIRARE DISEASESGROUP
Rx only

DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site


