Thioridazine Hydrochloride (thioridazine hydrochloride 25 mg) Dailymed
Generic: thioridazine hydrochloride is used for the treatment of Blood Coagulation Disorders Bone Marrow Diseases Coma Hypertension Hypotension Psychotic Disorders Schizophrenia
IMPRINT: M 61 100
SHAPE: round
COLOR: orange
All Imprints
thioridazine 25 mg oral tablet - m 58 25 round orange
thioridazine hydrochloride 50 mg - m 59 50 round orange
thioridazine hydrochloride 100 mg - m 61 100 round orange
thioridazine hydrochloride 10 mg - m 54 10 round orange
thioridazine hydrochloride 25 mg - m 58 25 round orange
Boxed Warning
Warning
Go PRO for all pill images
Warning
Thioridazine has been shown to prolong the QTc interval in a dose related manner, and drugs with this potential, including thioridazine, have been associated with Torsades de pointes type arrhythmias and sudden death. Due to its potential for significant, possibly life threatening, proarrhythmic effects, thioridazine should be reserved for use in the treatment of schizophrenic patients who fail to show an acceptable response to adequate courses of treatment with other antipsychotic drugs, either because of insufficient effectiveness or the inability to achieve an effective dose due to intolerable adverse effects from those drugs (see WARNINGS, CONTRAINDICATIONS, and INDICATIONS).
Increased Mortality in Elderly Patients with Dementia-Related Psychosis
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. Thioridazine hydrochloride is not approved for the treatment of patients with dementia-related psychosis (see WARNINGS).
Description
Thioridazine hydrochloride is 2-methylmercapto-10-[2-(N-methyl-2-piperidyl) ethyl] phenothiazine. Its structural formula, molecular weight and molecular formula are:
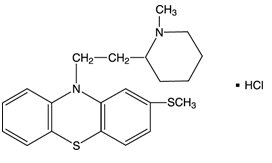
C21H26N2S2 • HCl M.Wt.: 407.05
Thioridazine hydrochloride, USP is available as tablets for oral administration containing 10 mg, 25 mg, 50 mg or 100 mg.
Each tablet for oral administration contains the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, FD&C Yellow No. 6 Aluminum Lake, hydroxypropyl cellulose, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, sodium lauryl sulfate and titanium dioxide.
Clinical Pharmacology
The basic pharmacological activity of thioridazine is similar to that of other phenothiazines, but is associated with minimal extrapyramidal stimulation.
However, thioridazine has been shown to prolong the QTc interval in a dose dependent fashion. This effect may increase the risk of serious, potentially fatal, ventricular arrhythmias, such as Torsades de pointes type arrhythmias. Due to this risk, thioridazine is indicated only for schizophrenic patients who have not been responsive to or cannot tolerate other antipsychotic agents (see WARNINGS and CONTRAINDICATIONS). However, the prescriber should be aware that thioridazine has not been systematically evaluated in controlled trials in treatment refractory schizophrenic patients and its efficacy in such patients is unknown.
Indications And Usage
Thioridazine hydrochloride tablets are indicated for the management of schizophrenic patients who fail to respond adequately to treatment with other antipsychotic drugs. Due to the risk of significant, potentially life threatening, proarrhythmic effects with thioridazine treatment, thioridazine hydrochloride tablets should be used only in patients who have failed to respond adequately to treatment with appropriate courses of other antipsychotic drugs, either because of insufficient effectiveness or the inability to achieve an effective dose due to intolerable adverse effects from those drugs. Consequently, before initiating treatment with thioridazine hydrochloride tablets, it is strongly recommended that a patient be given at least two trials, each with a different antipsychotic drug product, at an adequate dose, and for an adequate duration (see WARNINGS and CONTRAINDICATIONS).
However, the prescriber should be aware that thioridazine hydrochloride tablets have not been systematically evaluated in controlled trials in treatment refractory schizophrenic patients and its efficacy in such patients is unknown.
Contraindications
Thioridazine hydrochloride tablet use should be avoided in combination with other drugs that are known to prolong the QTc interval and in patients with congenital long QT syndrome or a history of cardiac arrhythmias.
Reduced cytochrome P450 2D6 isozyme activity drugs that inhibit this isozyme (e.g., fluoxetine and paroxetine) and certain other drugs (e.g., fluvoxamine, propranolol, and pindolol) appear to appreciably inhibit the metabolism of thioridazine. The resulting elevated levels of thioridazine would be expected to augment the prolongation of the QTc interval associated with thioridazine and may increase the risk of serious, potentially fatal, cardiac arrhythmias, such as Torsades de pointes type arrhythmias. Such an increased risk may result also from the additive effect of coadministering thioridazine with other agents that prolong the QTc interval. Therefore, thioridazine is contraindicated with these drugs as well as in patients, comprising about 7% of the normal population, who are known to have a genetic defect leading to reduced levels of activity of P450 2D6 (see WARNINGS and PRECAUTIONS).
In common with other phenothiazines, thioridazine is contraindicated in severe central nervous system depression or comatose states from any cause including drug induced central nervous system depression (see WARNINGS ). It should also be noted that hypertensive or hypotensive heart disease of extreme degree is a contraindication of phenothiazine administration.
Warnings
Increased Mortality in Elderly Patients with Dementia-Related Psychosis
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Thioridazine hydrochloride is not approved for the treatment of patients with dementia-related psychosis (see BOXED WARNING).
Potential for Proarrhythmic Effects
DUE TO THE POTENTIAL FOR SIGNIFICANT, POSSIBLY LIFE THREATENING, PROARRHYTHMIC EFFECTS WITH THIORIDAZINE TREATMENT, THIORIDAZINE SHOULD BE RESERVED FOR USE IN THE TREATMENT OF SCHIZOPHRENIC PATIENTS WHO FAIL TO SHOW AN ACCEPTABLE RESPONSE TO ADEQUATE COURSES OF TREATMENT WITH OTHER ANTIPSYCHOTIC DRUGS, EITHER BECAUSE OF INSUFFICIENT EFFECTIVENESS OR THE INABILITY TO ACHIEVE AN EFFECTIVE DOSE DUE TO INTOLERABLE ADVERSE EFFECTS FROM THOSE DRUGS. CONSEQUENTLY, BEFORE INITIATING TREATMENT WITH THIORIDAZINE, IT IS STRONGLY RECOMMENDED THAT A PATIENT BE GIVEN AT LEAST TWO TRIALS, EACH WITH A DIFFERENT ANTIPSYCHOTIC DRUG PRODUCT, AT AN ADEQUATE DOSE, AND FOR AN ADEQUATE DURATION. THIORIDAZINE HAS NOT BEEN SYSTEMATICALLY EVALUATED IN CONTROLLED TRIALS IN THE TREATMENT OF REFRACTORY SCHIZOPHRENIC PATIENTS AND ITS EFFICACY IN SUCH PATIENTS IS UNKNOWN.
A crossover study in nine healthy males comparing single doses of thioridazine 10 mg and 50 mg with placebo demonstrated a dose related prolongation of the QTc interval. The mean maximum increase in QTc interval following the 50 mg dose was about 23 msec; greater prolongation may be observed in the clinical treatment of unscreened patients.
Prolongation of the QTc interval has been associated with the ability to cause Torsades de pointes type arrhythmias, a potentially fatal polymorphic ventricular tachycardia, and sudden death. There are several published case reports of Torsades de pointes and sudden death associated with thioridazine treatment. A causal relationship between these events and thioridazine therapy has not been established but, given the ability of thioridazine to prolong the QTc interval, such a relationship is possible.
Certain circumstances may increase the risk of Torsades de pointes and/or sudden death in association with the use of drugs that prolong the QTc interval, including 1) bradycardia, 2) hypokalemia, 3) concomitant use of other drugs that prolong the QTc interval, 4) presence of congenital prolongation of the QT interval, and 5) for thioridazine in particular, its use in patients with reduced activity of P450 2D6 or its coadministration with drugs that may inhibit P450 2D6 or by some other mechanism interfere with the clearance of thioridazine (see CONTRAINDICATIONS and PRECAUTIONS).
It is recommended that patients being considered for thioridazine treatment have a baseline ECG performed and serum potassium levels measured. Serum potassium should be normalized before initiating treatment and patients with a QTc interval greater than 450 msec should not receive thioridazine treatment. It may also be useful to periodically monitor ECG’s and serum potassium during thioridazine treatment, especially during a period of dose adjustment. Thioridazine should be discontinued in patients who are found to have a QTc interval over 500 msec.
Patients taking thioridazine who experience symptoms that may be associated with the occurrence of Torsades de pointes (e.g., dizziness, palpitations, or syncope) may warrant further cardiac evaluation; in particular, Holter monitoring should be considered.
Tardive Dyskinesia
Tardive dyskinesia, a syndrome consisting of potentially irreversible, involuntary, dyskinetic movements may develop in patients treated with antipsychotic drugs. Although the prevalence of the syndrome appears to be highest among the elderly, especially elderly women, it is impossible to rely upon prevalence estimates to predict, at the inception of antipsychotic treatment, which patients are likely to develop the syndrome. Whether antipsychotic drug products differ in their potential to cause tardive dyskinesia is unknown.
Both the risk of developing the syndrome and the likelihood that it will become irreversible are believed to increase as the duration of treatment and the total cumulative dose of antipsychotic drugs administered to the patient increase. However, the syndrome can develop, although much less commonly, after relatively brief treatment periods at low doses.
There is no known treatment for established cases of tardive dyskinesia, although the syndrome may remit, partially or completely, if antipsychotic treatment is withdrawn. Antipsychotic treatment itself, however, may suppress (or partially suppress) the signs and symptoms of the syndrome and thereby may possibly mask the underlying disease process. The effect that symptomatic suppression has upon the long-term course of the syndrome is unknown.
Given these considerations, antipsychotics should be prescribed in a manner that is most likely to minimize the occurrence of tardive dyskinesia. Chronic antipsychotic treatment should generally be reserved for patients who suffer from a chronic illness that, 1) is known to respond to antipsychotic drugs, and, 2) for whom alternative, equally effective, but potentially less harmful treatments are not available or appropriate. In patients who do require chronic treatment, the smallest dose and the shortest duration of treatment producing a satisfactory clinical response should be sought. The need for continued treatment should be reassessed periodically.
If signs and symptoms of tardive dyskinesia appear in a patient on antipsychotics, drug discontinuation should be considered. However, some patients may require treatment despite the presence of the syndrome.
(For further information about the description of tardive dyskinesia and its clinical detection, please refer to the sections on Information for Patients and ADVERSE REACTIONS.)
It has been suggested in regard to phenothiazines in general, that people who have demonstrated a hypersensitivity reaction (e.g., blood dyscrasias, jaundice) to one may be more prone to demonstrate a reaction to others. Attention should be paid to the fact that phenothiazines are capable of potentiating central nervous system depressants (e.g., anesthetics, opiates, alcohol, etc.) as well as atropine and phosphorus insecticides. Physicians should carefully consider benefit versus risk when treating less severe disorders.
Reproductive studies in animals and clinical experience to date have failed to show a teratogenic effect with thioridazine. However, in view of the desirability of keeping the administration of all drugs to a minimum during pregnancy, thioridazine should be given only when the benefits derived from treatment exceed the possible risks to mother and fetus.
Pregnancy
Neonates exposed to antipsychotic drugs, during the third trimester of pregnancy are at risk for extrapyramidal and/or withdrawal symptoms following delivery. There have been reports of agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress and feeding disorder in these neonates. These complications have varied in severity; while in some cases symptoms have been self-limited, in other cases neonates have required intensive care unit support and prolonged hospitalization.
Thioridazine hydrochloride should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Neuroleptic Malignant Syndrome (NMS)
A potentially fatal symptom complex sometimes referred to as Neuroleptic Malignant Syndrome (NMS) has been reported in association with antipsychotic drugs. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status, and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmias).
The diagnostic evaluation of patients with this syndrome is complicated. In arriving at a diagnosis, it is important to identify cases where the clinical presentation includes both serious medical illness (e.g., pneumonia, systemic infection, etc.) and untreated or inadequately treated extrapyramidal signs and symptoms (EPS). Other important considerations in the differential diagnosis include central anticholinergic toxicity, heat stroke, drug fever, and primary central nervous system (CNS) pathology.
The management of NMS should include, 1) immediate discontinuation of antipsychotic drugs and other drugs not essential to concurrent therapy, 2) intensive symptomatic treatment and medical monitoring, and 3) treatment of any concomitant serious medical problems for which specific treatments are available. There is no general agreement about specific pharmacological treatment regimens for uncomplicated NMS.
If a patient requires antipsychotic drug treatment after recovery from NMS, the potential reintroduction of drug therapy should be carefully considered. The patient should be carefully monitored, since recurrences of NMS have been reported.
Falls
Thioridazine hydrochloride tablets may cause somnolence, postural hypotension, motor and sensory instability, which may lead to falls and, consequently, fractures or other injuries. For patients with diseases, conditions, or medications that could exacerbate these effects, complete fall risk assessments when initiating antipsychotic treatment and recurrently for patients on long-term antipsychotic therapy.
Central Nervous System Depressants
As in the case of other phenothiazines, thioridazine is capable of potentiating central nervous system depressants (e.g., alcohol, anesthetics, barbiturates, narcotics, opiates, other psychoactive drugs, etc.) as well as atropine and phosphorus insecticides. Severe respiratory depression and respiratory arrest have been reported when a patient was given a phenothiazine and a concomitant high dose of a barbiturate.
Precautions
Leukopenia and/or agranulocytosis and convulsive seizures have been reported but are infrequent. In schizophrenic patients with epilepsy, anticonvulsant medication should be maintained during treatment with thioridazine. Pigmentary retinopathy, which has been observed primarily in patients taking larger than recommended doses, is characterized by diminution of visual acuity, brownish coloring of vision, and impairment of night vision; examination of the fundus discloses deposits of pigment. The possibility of this complication may be reduced by remaining within the recommended limits of dosage.
Where patients are participating in activities requiring complete mental alertness (e.g., driving) it is advisable to administer the phenothiazines cautiously and to increase the dosage gradually. Female patients appear to have a greater tendency to orthostatic hypotension than male patients. The administration of epinephrine should be avoided in the treatment of drug-induced hypotension in view of the fact that phenothiazines may induce a reversed epinephrine effect on occasion. Should a vasoconstrictor be required, the most suitable are levarterenol and phenylephrine.
Antipsychotic drugs elevate prolactin levels; the elevation persists during chronic administration. Tissue culture experiments indicate that approximately one-third of human breast cancers are prolactin dependent in vitro, a factor of potential importance if the prescription of these drugs is contemplated in a patient with a previously detected breast cancer. Although disturbances such as galactorrhea, amenorrhea, gynecomastia, and impotence have been reported, the clinical significance of elevated serum prolactin levels is unknown for most patients. An increase in mammary neoplasms has been found in rodents after chronic administration of neuroleptic drugs. Some epidemiologic studies have indicated a potential association between chronic administration of prolactin-increasing antipsychotics and breast cancer.
Drug Interactions
Reduced cytochrome P450 2D6 isozyme activity, drugs which inhibit this isozyme (e.g., fluoxetine and paroxetine), and certain other drugs (e.g., fluvoxamine, propranolol, and pindolol) appear to appreciably inhibit the metabolism of thioridazine. The resulting elevated levels of thioridazine would be expected to augment the prolongation of the QTc interval associated with thioridazine and may increase the risk of serious, potentially fatal, cardiac arrhythmias, such as Torsades de pointes type arrhythmias. Such an increased risk may result also from the additive effect of coadministering thioridazine with other agents that prolong the QTc interval. Therefore, thioridazine is contraindicated with these drugs as well as in patients, comprising about 7% of the normal population, who are known to have a genetic defect leading to reduced levels of activity of P450 2D6 (see WARNINGS and CONTRAINDICATIONS).
In a study of 19 healthy male subjects, which included 6 slow and 13 rapid hydroxylators of debrisoquin, a single 25 mg oral dose of thioridazine produced a 2.4-fold higher Cmax and a 4.5-fold higher AUC for thioridazine in the slow hydroxylators compared to rapid hydroxylators. The rate of debrisoquin hydroxylation is felt to depend on the level of cytochrome P450 2D6 isozyme activity. Thus, this study suggests that drugs that inhibit P450 2D6 or the presence of reduced activity levels of this isozyme will produce elevated plasma levels of thioridazine. Therefore, the coadministration of drugs that inhibit P450 2D6 with thioridazine and the use of thioridazine in patients known to have reduced activity of P450 2D6 are contraindicated.
Fluvoxamine
The effect of fluvoxamine (25 mg b.i.d. for one week) on thioridazine steady-state concentration was evaluated in ten male inpatients with schizophrenia. Concentrations of thioridazine and its two active metabolites, mesoridazine and sulforidazine, increased 3-fold following coadministration of fluvoxamine. Fluvoxamine and thioridazine should not be coadministered.
Propranolol
Concurrent administration of propranolol (100 mg to 800 mg daily) has been reported to produce increases in plasma levels of thioridazine (approximately 50% to 400%) and its metabolites (approximately 80% to 300%). Propranolol and thioridazine should not be coadministered.
Pindolol
Concurrent administration of pindolol and thioridazine have resulted in moderate, dose related increases in the serum levels of thioridazine and two of its metabolites, as well as higher than expected serum pindolol levels. Pindolol and thioridazine should not be coadministered.
There are no studies of the coadministration of thioridazine and other drugs that prolong the QTc interval. However, it is expected that such coadministration would produce additive prolongation of the QTc interval and, thus, such use is contraindicated.
Information for Patients
Patients should be informed that thioridazine has been associated with potentially fatal heart rhythm disturbances. The risk of such events may be increased when certain drugs are given together with thioridazine. Therefore, patients should inform the prescriber that they are receiving thioridazine treatment before taking any new medication.
Given the likelihood that some patients exposed chronically to antipsychotics will develop tardive dyskinesia, it is advised that all patients in whom chronic use is contemplated be given, if possible, full information about this risk. The decision to inform patients and/or their guardians must obviously take into account the clinical circumstances and the competency of the patient to understand the information provided.
Pediatric Use
See DOSAGE AND ADMINISTRATION: Pediatric Patients.
Leukopenia, Neutropenia and Agranulocytosis
In clinical trial and post-marketing experience, events of leukopenia/neutropenia and agranulocytosis have been reported temporally related to antipsychotic agents.
Possible risk factors for leukopenia/neutropenia include preexisting low white blood cell count (WBC) and history of drug induced leukopenia/neutropenia. Patients with a preexisting low WBC or a history of drug induced leukopenia/neutropenia should have their complete blood count (CBC) monitored frequently during the first few months of therapy and should discontinue Thioridazine Hydrochloride Tablets at the first sign of a decline in WBC in the absence of other causative factors.
Patients with neutropenia should be carefully monitored for fever or other symptoms or signs of infection and treated promptly if such symptoms occur. Patients with severe neutropenia (absolute neutrophil count < 1000/mm3) should discontinue Thioridazine Hydrochloride Tablets and have their WBC followed until recovery.
Adverse Reactions
In the recommended dosage ranges with thioridazine hydrochloride most side effects are mild and transient.
Central Nervous System
Drowsiness may be encountered on occasion, especially where large doses are given early in treatment. Generally, this effect tends to subside with continued therapy or a reduction in dosage. Pseudoparkinsonism and other extrapyramidal symptoms may occur but are infrequent. Nocturnal confusion, hyperactivity, lethargy, psychotic reactions, restlessness, and headache have been reported but are extremely rare.
Autonomic Nervous System
Dryness of mouth, blurred vision, constipation, nausea, vomiting, diarrhea, nasal stuffiness, and pallor have been seen.
Endocrine System
Galactorrhea, breast engorgement, amenorrhea, inhibition of ejaculation, and peripheral edema have been described.
Skin
Dermatitis and skin eruptions of the urticarial type have been observed infrequently. Photosensitivity is extremely rare.
Cardiovascular System
Thioridazine produces a dose related prolongation of the QTc interval, which is associated with the ability to cause Torsades de pointes type arrhythmias, a potentially fatal polymorphic ventricular tachycardia, and sudden death (see WARNINGS). Both Torsades de pointes type arrhythmias and sudden death have been reported in association with thioridazine. A causal relationship between these events and thioridazine therapy has not been established but, given the ability of thioridazine to prolong the QTc interval, such a relationship is possible. Other ECG changes have been reported (see Phenothiazine Derivatives: Cardiovascular Effects).
Other
Rare cases described as parotid swelling have been reported following administration of thioridazine.
Post Introduction Reports
These are voluntary reports of adverse events temporally associated with thioridazine that were received since marketing, and there may be no causal relationship between thioridazine use and these events: priapism.
Phenothiazine Derivatives
It should be noted that efficacy, indications, and untoward effects have varied with the different phenothiazines. It has been reported that old age lowers the tolerance for phenothiazines. The most common neurological side effects in these patients are parkinsonism and akathisia. There appears to be an increased risk of agranulocytosis and leukopenia in the geriatric population. The physician should be aware that the following have occurred with one or more phenothiazines and should be considered whenever one of these drugs is used:
Autonomic Reactions:Â Miosis, obstipation, anorexia, paralytic ileus.
Cutaneous Reactions:Â Erythema, exfoliative dermatitis, contact dermatitis.
Blood Dyscrasias:Â Agranulocytosis, leukopenia, eosinophilia, thrombocytopenia, anemia, aplastic anemia, pancytopenia.
Allergic Reactions:Â Fever, laryngeal edema, angioneurotic edema, asthma.
Hepatotoxicity:Â Jaundice, biliary stasis.
Cardiovascular Effects:Â Changes in the terminal portion of the electrocardiogram to include prolongation of the QT interval, depression and inversion of the T wave, and the appearance of a wave tentatively identified as a bifid T wave or a U wave have been observed in patients receiving phenothiazines, including thioridazine. To date, these appear to be due to altered repolarization, not related to myocardial damage, and reversible. Nonetheless, significant prolongation of the QT interval has been associated with serious ventricular arrhythmias and sudden death (see WARNINGS). Hypotension, rarely resulting in cardiac arrest, has been reported.
Extrapyramidal Symptoms:Â Akathisia, agitation, motor restlessness, dystonic reactions, trismus, torticollis, opisthotonus, oculogyric crises, tremor, muscular rigidity, akinesia.
Tardive Dyskinesia:Â Chronic use of antipsychotics may be associated with the development of tardive dyskinesia. The salient features of this syndrome are described in the WARNINGS section and subsequently.
The syndrome is characterized by involuntary choreoathetoid movements which variously involve the tongue, face, mouth, lips, or jaw (e.g., protrusion of the tongue, puffing of cheeks, puckering of the mouth, chewing movements), trunk, and extremities. The severity of the syndrome and the degree of impairment produced vary widely.
The syndrome may become clinically recognizable either during treatment, upon dosage reduction, or upon withdrawal of treatment. Movements may decrease in intensity and may disappear altogether if further treatment with antipsychotics is withheld. It is generally believed that reversibility is more likely after short rather than long-term antipsychotic exposure. Consequently, early detection of tardive dyskinesia is important. To increase the likelihood of detecting the syndrome at the earliest possible time, the dosage of antipsychotic drug should be reduced periodically (if clinically possible) and the patient observed for signs of the disorder. This maneuver is critical, for antipsychotic drugs may mask the signs of the syndrome.
Neuroleptic Malignant Syndrome (NMS):Â Chronic use of antipsychotics may be associated with the development of Neuroleptic Malignant Syndrome. The salient features of this syndrome are described in the WARNINGS section and subsequently. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status, and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmias).
Endocrine Disturbances:Â Menstrual irregularities, altered libido, gynecomastia, lactation, weight gain, edema. False positive pregnancy tests have been reported.
Urinary Disturbances:Â Retention, incontinence.
Others:Â Hyperpyrexia. Behavioral effects suggestive of a paradoxical reaction have been reported. These include exclient, bizarre dreams, aggravation of psychoses, and toxic confusional states. More recently, a peculiar skin-eye syndrome has been recognized as a side effect following long-term treatment with phenothiazines. This reaction is marked by progressive pigmentation of areas of the skin or conjunctiva and/or accompanied by discoloration of the exposed sclera and cornea. Opacities of the anterior lens and cornea described as irregular or stellate in shape have also been reported. Systemic lupus erythematosus-like syndrome.
Overdosage
Many of the symptoms observed are extensions of the side effects described under ADVERSE REACTIONS. Thioridazine can be toxic in overdose, with cardiac toxicity being of particular concern. Frequent ECG and vital sign monitoring of overdosed patients is recommended. Observation for several days may be required because of the risk of delayed effects.
Signs and Symptoms
Effects and clinical complications of acute overdose involving phenothiazines may include:
Cardiovascular:Â Cardiac arrhythmias, hypotension, shock, ECG changes, increased QT and PR intervals, non-specific ST and T wave changes, bradycardia, sinus tachycardia, atrioventricular block, ventricular tachycardia, ventricular fibrillation, Torsades de pointes, myocardial depression.
Central Nervous System:Â Sedation, extrapyramidal effects, confusion, agitation, hypothermia, hyperthermia, restlessness, seizures, areflexia, coma.
Autonomic Nervous System:Â Mydriasis, miosis, dry skin, dry mouth, nasal congestion, urinary retention, blurred vision.
Respiratory:Â Respiratory depression, apnea, pulmonary edema.
Gastrointestinal:Â Hypomotility, constipation, ileus.
Renal:Â Oliguria, uremia.
Toxic dose and blood concentration ranges for the phenothiazines have not been firmly established. It has been suggested that the toxic blood concentration range for thioridazine begins at 1 mg/dL, and 2 to 8 mg/dL is the lethal concentration range.
Treatment
An airway must be established and maintained. Adequate oxygenation and ventilation must be ensured.
Cardiovascular monitoring should commence immediately and should include continuous electrocardiographic monitoring to detect possible arrhythmias. Treatment may include one or more of the following therapeutic interventions: correction of electrolyte abnormalities and acid-base balance, lidocaine, phenytoin, isoproterenol, ventricular pacing, and defibrillation. Disopyramide, procainamide, and quinidine may produce additive QT-prolonging effects when administered to patients with acute overdosage of thioridazine and should be avoided (see WARNINGS and CONTRAINDICATIONS). Caution must be exercised when administering lidocaine, as it may increase the risk of developing seizures.
Treatment of hypotension may require intravenous fluids and vasopressors. Phenylephrine, levarterenol, or metaraminol are the appropriate pressor agents for use in the management of refractory hypotension. The potent α adrenergic blocking properties of the phenothiazines makes the use of vasopressors with mixed α and ß adrenergic agonist properties inappropriate, including epinephrine and dopamine. Paradoxical vasodilation may result. In addition, it is reasonable to expect that the α adrenergic-blocking properties of bretylium might be additive to those of thioridazine, resulting in problematic hypotension.
In managing overdosage, the physician should always consider the possibility of multiple drug involvement. Gastric lavage and repeated doses of activated charcoal should be considered. Induction of emesis is less preferable to gastric lavage because of the risk of dystonia and the potential for aspiration of vomitus. Emesis should not be induced in patients expected to deteriorate rapidly, or those with impaired consciousness.
Acute extrapyramidal symptoms may be treated with diphenhydramine hydrochloride or benztropine mesylate.
Avoid the use of barbiturates when treating seizures, as they may potentiate phenothiazine-induced respiratory depression.
Forced diuresis, hemoperfusion, hemodialysis and manipulation of urine pH are of unlikely benefit in the treatment of phenothiazine overdose due to their large volume of distribution and extensive plasma protein binding.
Up-to-date information about the treatment of overdose can often be obtained from a certified Regional Poison Control Center. Telephone numbers of certified Regional Poison Control Centers are uled in the Physicians’ Desk Reference®**.
Dosage And Administration
Since thioridazine hydrochloride tablets are associated with a dose related prolongation of the QTc interval, which is a potentially life threatening event, its use should be reserved for schizophrenic patients who fail to respond adequately to treatment with other antipsychotic drugs. Dosage must be individualized and the smallest effective dosage should be determined for each patient (see INDICATIONS and WARNINGS).
Adults
The usual starting dose for adult schizophrenic patients is 50 mg to 100 mg three times a day, with a gradual increment to a maximum of 800 mg daily if necessary. Once effective control of symptoms has been achieved, the dosage may be reduced gradually to determine the minimum maintenance dose. The total daily dosage ranges from 200 mg to 800 mg, divided into two to four doses.
Pediatric Patients
For pediatric patients with schizophrenia who are unresponsive to other agents, the recommended initial dose is 0.5 mg/kg/day given in divided doses. Dosage may be increased gradually until optimum therapeutic effect is obtained or the maximum dose of 3 mg/kg/day has been reached.
How Supplied
Thioridazine Hydrochloride Tablets, USP are available containing 10 mg, 25 mg, 50 mg or 100 mg of thioridazine hydrochloride, USP.
The 10 mg tablets are orange film-coated, round, unscored tablets debossed with M over 54 on one side and 10 on the other side. They are available as follows:
NDC 0378-0612-01bottles of 100 tablets
The 25 mg tablets are orange film-coated, round, unscored tablets debossed with M over 58 on one side and 25 on the other side. They are available as follows:
NDC 0378-0614-01bottles of 100 tablets
The 50 mg tablets are orange film-coated, round, unscored tablets debossed with M over 59 on one side and 50 on the other side. They are available as follows:
NDC 0378-0616-01bottles of 100 tablets
The 100 mg tablets are orange film-coated, round, unscored tablets debossed with M over 61 on one side and 100 on the other side. They are available as follows:
NDC 0378-0618-01bottles of 100 tablets
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Protect from light.
Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
**Trademark of Medical Economics Company, Inc.
Manufactured for: Mylan Pharmaceuticals Inc. Morgantown, WV 26505 U.S.A.
Manufactured by: Mylan Laboratories Limited Hyderabad — 500 096, India
750XXXXX
Revised: 9/2024MX:THIO:RX
Principal Display Panel 10 Mg
NDC 0378-0612-01
ThioridazineHydrochlorideTablets, USP10 mg
Rx only   100 Tablets
Each film-coated tablet contains:Thioridazine hydrochloride, USP 10 mg
Usual Dosage: See accompanyingprescribing information.
Keep this and all medication out ofthe reach of children.
Store at 20° to 25°C (68° to 77°F). [SeeUSP Controlled Room Temperature.]
Protect from light.
Manufactured for: Mylan Pharmaceuticals Inc. Morgantown, WV 26505 U.S.A.
Made in India
RMX0612A
Dispense in a tight, light-resistantcontainer as defined in the USPusing a child-resistant closure.
Keep container tightly closed.
Code No.: MH/DRUGS/25/NKD/89
Principal Display Panel 25 Mg
NDC 0378-0614-01
ThioridazineHydrochlorideTablets, USP25 mg
Rx only   100 Tablets
Each film-coated tablet contains:Thioridazine hydrochloride, USP 25 mg
Usual Dosage: See accompanyingprescribing information.
Keep this and all medication out ofthe reach of children.
Store at 20° to 25°C (68° to 77°F). [SeeUSP Controlled Room Temperature.]
Protect from light.
Manufactured for: Mylan Pharmaceuticals Inc. Morgantown, WV 26505 U.S.A.
Made in India
RMX0614A
Dispense in a tight, light-resistantcontainer as defined in the USPusing a child-resistant closure.
Keep container tightly closed.
Code No.: MH/DRUGS/25/NKD/89

Principal Display Panel 50 Mg
NDC 0378-0616-01
ThioridazineHydrochlorideTablets, USP50 mg
Rx only   100 Tablets
Each film-coated tablet contains:Thioridazine hydrochloride, USP 50 mg
Usual Dosage: See accompanyingprescribing information.
Keep this and all medication out ofthe reach of children.
Store at 20° to 25°C (68° to 77°F). [SeeUSP Controlled Room Temperature.]
Protect from light.
Manufactured for: Mylan Pharmaceuticals Inc. Morgantown, WV 26505 U.S.A.
Made in India
RMX0616A
Dispense in a tight, light-resistantcontainer as defined in the USPusing a child-resistant closure.
Keep container tightly closed.
Code No.: MH/DRUGS/25/NKD/89

Principal Display Panel 100 Mg
NDC 0378-0618-01
ThioridazineHydrochlorideTablets, USP100 mg
Rx only   100 Tablets
Each film-coated tablet contains:Thioridazine hydrochloride, USP 100 mg
Usual Dosage: See accompanyingprescribing information.
Keep this and all medication out ofthe reach of children.
Store at 20° to 25°C (68° to 77°F). [SeeUSP Controlled Room Temperature.]
Protect from light.
Manufactured for: Mylan Pharmaceuticals Inc. Morgantown, WV 26505 U.S.A.
Made in India
RMX0618A
Dispense in a tight, light-resistantcontainer as defined in the USPusing a child-resistant closure.
Keep container tightly closed.
Code No.: MH/DRUGS/25/NKD/89
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site


