TRAMADOL HYDROCHLORIDE (tramadol hydrochloride 50 mg) Dailymed
Generic: tramadol hydrochloride is used for the treatment of Opioid-Related Disorders Pain
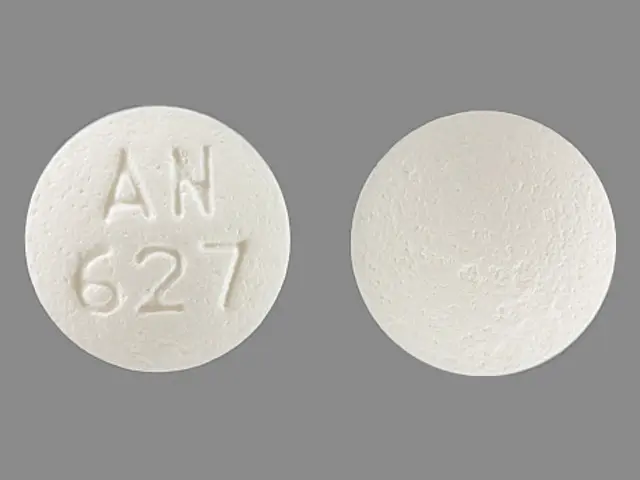
IMPRINT: AN 627
SHAPE: round
COLOR: white
All Imprints
tramadol hydrochloride 50 mg - an 627 round white
tramadol hydrochloride 50 mg oral tablet - an 627 round white
Go PRO for all pill images
Description Section
Each capsule contains:
Tramadol Hydrochloride, USP ..........50 mg
Description Section
Usual Dosage:  For dosage and other prescribing
information, see accompanying insert.
Storage And Handling Section
Store at 20 to 25 C (68 to 77 F); excursions
permitted to 15 to 30 C (59 to 86 F)[See
USP Controlled Room Temperature]
Dispense in a tight container as defined in
the USP.
Keep out of reach of children.
Description Section
DESCRIPTION
Tramadol hydrochloride tablets, USP are a centrally acting analgesic.
Clinical Pharmacology Section
CLINICAL PHARMACOLOGY
PHARMACODYNAMICS    Tramadol Hydrochloride contains tramadol, a centrally acting synthetic opoid
analgesic.
Clinical Studies Section
CLINICAL STUDIES
Tramadol hydrochloride has been given in single oral doses pf 50, 75 and 100 mg to patients with pain
following surgical procedures and pain following oral surgery (extraction if impacted molars).
Indications & Usage Section
INDICATIONS AND USAGE
Tramadol hydrochloride tablets, USP are indicated for the management of moderate to
moderately severe pain in adults.
Contraindications Section
CONTRAINDICATIONS
Tramadol hydrochloride tablets, USP should not be administered to patients who have previously
demonstrated hypersensitivity to tramadol, any other component of this product or opoids.
Warnings Section
WARNINGS
Seizure Risk   Seizures have been reported in patients receiving Tramadol hydrochloride within the
recommended dosage range.
Precautions Section
PRECAUTIONS
Acute Abdominal Conditions   The administration of tramadol hydrochloride may complicate the clinical assessment
of patients with acute abdominal conditions
Adverse Reactions Section
ADVERSE REACTIONS
Tramadol hydrochloride was administered to 550 patients during the double-blind or open-label
extension periods in U.S. clinical studies of chronic nonmalignant pain.
Drug Abuse And Dependence Section
DRUG ABUSE AND DEPENDENCE.
Abuse   Tramadol has mu-opoid agonist activity.
Overdosage Section
OVERDOSAGE
Acute overdosage with tramadol can be manifested by respiratory depression, somnolence progressing to
stupor or coma, skeletal muscle flaccidity, cold and clammy skin, constricted pupils, seizures,
bradycardia, hypotension, cardiac arrest, and death.
Dosage & Administration Section
DOSAGE AND ADMINISTRATION
Adults (17 years of age and older)    For patients with moderate to moderately severe chronic pain not
requiringrapid onset of analgesic effect, the tolerability of tramadol hydrochloride, USP can be improved by
initiating therapy with a titration regimen:
Package Label.principal Display Panel
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site