Vetoryl (trilostane 60 mg) Dailymed
Generic: trilostane
Go PRO for all pill images
Adrenocortical suppressant for oral use in dogs only.
Caution:
Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
Description:
VETORYL Capsules are available in 6 sizes (5, 10, 20, 30, 60 and 120 mg) for oral administration based on body weight. Trilostane (4α,5α-epoxy-17β-hydroxy-3-oxoandrostane-2α-carbonitrile) is an orally active synthetic steroid analogue that selectively inhibits 3 β-hydroxysteroid dehydrogenase in the adrenal cortex, thereby inhibiting the conversion of pregnenolone to progesterone. This inhibition blocks production of glucocorticoids and to a lesser extent, mineralocorticoids and sex hormones while steroid precursor levels increase. The structural formula is:
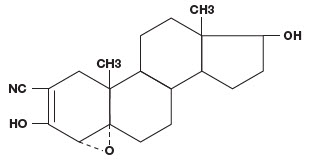
Indications:
VETORYL Capsules are indicated for the treatment of pituitary-dependent and adrenal-dependent hyperadrenocorticism in dogs.
Dosage And Administration:
Always provide the Client Information Sheet with prescription (see INFORMATION FOR DOG OWNERS ).
1. Starting dose
The starting dose for the treatment of hyperadrenocorticism in dogs is 1-3 mg/lb (2.2-6.7 mg/kg) once a day. Start with the lowest possible dose based on body weight and available combinations of capsule sizes. VETORYL Capsules should be administered with food.
2. Action at 10-14 day evaluation (Table 1)
After approximately 10-14 days at this dose, re-examine the dog and conduct a 4-6 hour post-dosing ACTH stimulation test and serum biochemical tests (with particular attention to electrolytes, and renal and hepatic function). If physical examination is acceptable, take action according to Table 1.
Owners should be instructed to stop therapy and contact their veterinarian immediately in the event of adverse reactions such as vomiting, diarrhea, lethargy, poor/reduced appetite, weakness, collapse or any other unusual developments. If these clinical signs are observed, conduct an ACTH stimulation test and serum biochemical tests (with particular attention to electrolytes, and renal and hepatic function).
Table 1: Action at 10-14 day evaluation Post-ACTH serum cortisol Action µg/dL nmol/L < 1.45 < 40 Stop treatment. Re-start at a decreased dose 1.45 to 5.4 40 to 150 Continue on same dose > 5.4 to 9.1 > 150 to 250 EITHER: Continue on current dose if clinical signs are well controlledOR: Increase dose if clinical signs of hyperadrenocorticism are still evident Combinations of capsule sizes should be used to slowly increase the once daily dose. > 9.1 > 250 Increase initial dose 3. Individual dose adjustments and close monitoring are essential
Re-examine and conduct an ACTH stimulation test and serum biochemical tests (with particular attention to electrolytes, and renal and hepatic function) 10-14 days after every dose alteration. Care must be taken during dose increases to monitor the dog's clinical signs.
Once daily administration is recommended. However, if clinical signs are not controlled for the full day, twice daily dosing may be needed. To switch from a once daily dose to a twice daily dose, the total daily dose should be divided into 2 portions given 12 hours apart. It is not necessary for the portions to be equal. If applicable, the larger dose should be administered in the morning and the smaller dose in the evening. For example, a dog receiving 90 mg would receive 60 mg in the morning, and 30 mg in evening.
4. Long term monitoring
Once an optimum dose of VETORYL Capsules has been reached, re-examine the dog at 30 days, 90 days and every 3 months thereafter. At a minimum, this monitoring should include:
- A thorough history and physical examination.
- An ACTH stimulation test (conducted 4-6 hours after VETORYL Capsule administration) - a post-ACTH stimulation test resulting in a cortisol of < 1.45 μg/dL (< 40 nmol/L), with or without electrolyte abnormalities, may precede the development of clinical signs of hypoadrenocorticism.
- Serum biochemical tests (with particular attention to electrolytes, and renal and hepatic function).
Good control is indicated by favorable clinical signs as well as post-ACTH serum cortisol of 1.45-9.1 μg/dL (40-250 nmol/L).
If the ACTH stimulation test is < 1.45 µg/dL (< 40 nmol/L) and/or if electrolyte imbalances characteristic of hypoadrenocorticism (hyperkalemia and hyponatremia) are found, VETORYL Capsules should be temporarily discontinued until recurrence of clinical signs consistent with hyperadrenocorticism and ACTH stimulation test results return to normal (1.45-9.1 µg/dL or 40-250 nmol/L).
VETORYL Capsules may then be re-introduced at a lower dose.
Contraindications:
The use of VETORYL Capsules is contraindicated in dogs that have demonstrated hypersensitivity to trilostane.
Do not use VETORYL Capsules in animals with primary hepatic disease or renal insufficiency (See WARNINGS and PRECAUTIONS ).
Do not use in pregnant dogs. Studies conducted with trilostane in laboratory animals have shown teratogenic effects and early pregnancy loss.
Warnings:
Hypoadrenocorticism can develop at any dose of VETORYL Capsules. In some cases, it may take months for adrenal function to return and some dogs never regain adequate adrenal function.
All dogs should undergo a thorough history and physical examination before initiation of therapy with VETORYL Capsules. Other conditions, such as primary hepatic and/or renal disease should be considered when the patient is exhibiting signs of illness in addition to signs of hyperadrenocorticism (e.g. vomiting, diarrhea, poor/reduced appetite, weight loss, and lethargy). Appropriate laboratory tests to establish hematological and serum biochemical baseline data prior to, and periodically during, administration of VETORYL Capsules should be considered.
Owners should be advised to discontinue therapy immediately and contact their veterinarian if signs of potential drug toxicity are observed (see INFORMATION FOR DOG OWNERS, DOSAGE AND ADMINISTRATION, PRECAUTIONS, ADVERSE REACTIONS, ANIMAL SAFETY and POST-APPROVAL EXPERIENCE).
In case of overdosage, symptomatic treatment of hypoadrenocorticism with corticosteroids, mineralocorticoids and intravenous fluids may be required.
Angiotensin converting enzyme (ACE) inhibitors should be used with caution with VETORYL Capsules, as both drugs have aldosterone-lowering effects which may be additive, impairing the patient's ability to maintain normal electrolytes, blood volume and renal perfusion. Potassium sparing diuretics (e.g. spironolactone) should not be used with VETORYL Capsules as both drugs have the potential to inhibit aldosterone, increasing the likelihood of hyperkalemia.
Human Warnings:
Keep out of reach of children. Not for human use.
Wash hands after use. Do not empty capsule contents and do not attempt to divide the capsules. Do not handle the capsules if pregnant or if trying to conceive. Trilostane is associated with teratogenic effects and early pregnancy loss in laboratory animals. In the event of accidental ingestion/overdose, seek medical advice immediately and take the labeled container with you.
Precautions:
Mitotane (o,p'-DDD) treatment will reduce adrenal function. Experience in foreign markets suggests that when mitotane therapy is stopped, an interval of at least one month should elapse before the introduction of VETORYL Capsules. It is important to wait for both the recurrence of clinical signs consistent with hyperadrenocorticism, and a post-ACTH cortisol level of > 9.1 μg/dL (> 250 nmol/L) before treatment with VETORYL Capsules is initiated. Close monitoring of adrenal function is advised, as dogs previously treated with mitotane may be more responsive to the effects of VETORYL Capsules.
The use of VETORYL Capsules will not affect the adrenal tumor itself. Adrenalectomy should be considered as an option for cases that are good surgical candidates. The safe use of this drug has not been evaluated in lactating dogs and males intended for breeding.
Adverse Reactions:
The most common adverse reactions reported are poor/reduced appetite, vomiting, lethargy/dullness, diarrhea, and weakness. Occasionally, more serious reactions, including severe depression, hemorrhagic diarrhea, collapse, hypoadrenocortical crisis or adrenal necrosis/rupture may occur, and may result in death.
In a US field study with 107 dogs, adrenal necrosis/rupture (two dogs) and hypoadrenocorticism (two dogs) were the most severe adverse reactions in the study. One dog died suddenly of adrenal necrosis, approximately one week after starting trilostane therapy. One dog developed an adrenal rupture, believed to be secondary to adrenal necrosis, approximately six weeks after starting trilostane therapy. This dog responded to trilostane discontinuation and supportive care.
Two dogs developed hypoadrenocorticism during the study. These two dogs had clinical signs consistent with hypoadrenocorticism (lethargy, anorexia, collapse) and post-ACTH cortisol levels ≤ 0.3 μg/dL. Both dogs responded to trilostane discontinuation and supportive care, and one dog required continued treatment for hypoadrenocorticism (glucocorticoids and mineralocorticoids) after the acute presentation.
Additional adverse reactions were observed in 93 dogs. The most common of these included diarrhea (31 dogs), lethargy (30 dogs), inappetence/anorexia (27 dogs), vomiting (28 dogs), musculoskeletal signs (lameness, worsening of degenerative joint disease) (25 dogs), urinary tract infection (UTI)/hematuria (17 dogs), shaking/shivering (10 dogs), otitis externa (8 dogs), respiratory signs (coughing, congestion) (7 dogs), and skin/coat abnormality (seborrhea, pruritus) (8 dogs).
Five dogs died or were euthanized during the study (one dog secondary to adrenal necrosis, discussed above, two dogs due to progression of pre-existing congestive heart failure, one dog due to progressive central nervous system signs, and one dog due to cognitive decline leading to inappropriate elimination). In addition to the two dogs with adrenal necrosis/rupture and the two dogs with hypoadrenocorticism, an additional four dogs were removed from the study as a result of possible trilostane-related adverse reactions, including collapse, lethargy, inappetence, and trembling. Complete blood counts conducted pre- and post-treatment revealed a statistically significant (p <0.005) reduction in red cell variables (HCT, HGB, and RBC), but the mean values remained within the normal range. Additionally, approximately 10% of the dogs had elevated BUN values (≥ 40 mg/dL) in the absence of concurrent creatinine elevations. In general, these dogs were clinically normal at the time of the elevated BUN.
In a long term follow-up study of dogs in the US effectiveness study, the adverse reactions were similar to the short term study. Vomiting, diarrhea and general gastrointestinal signs were most commonly observed. Lethargy, inappetence/anorexia, heart murmur or cardiopulmonary signs, inappropriate urination/incontinence, urinary tract infections or genitourinary disease, and neurological signs were reported. Included in the US follow-up study were 14 deaths, three of which were possibly related to trilostane. Eleven dogs died or were euthanized during the study for a variety of conditions considered to be unrelated to or to have an unknown relationship with administration of trilostane.
In two UK field studies with 75 dogs, the most common adverse reactions seen were vomiting, lethargy, diarrhea/loose stools, and anorexia. Other adverse reactions included: nocturia, corneal ulcer, cough, persistent estrus, vaginal discharge and vulvar swelling in a spayed female, hypoadrenocorticism, electrolyte imbalance (elevated potassium with or without decreased sodium), collapse and seizure, shaking, muscle tremors, constipation, scratching, weight gain, and weight loss. One dog died of congestive heart failure and another died of pulmonary thromboembolism. Three dogs were euthanized during the study. Two dogs had renal failure and another had worsening arthritis and deterioration of appetite.
In a long term follow-up of dogs included in the UK field studies, the following adverse reactions were seen: hypoadrenocortical episode (including syncope, tremor, weakness, and vomiting), hypoadrenocortical crisis or renal failure (including azotemia, vomiting, dehydration, and collapse), chronic intermittent vaginal discharge, hemorrhagic diarrhea, occasional vomiting, and distal limb edema. Signs of hypoadrenocorticism were usually reversible after withdrawal of the drug, but may be permanent. One dog discontinued VETORYL Capsules and continued to have hypoadrenocorticism when evaluated a year later. Included in the follow-up were reports of deaths, at least 5 of which were possibly related to use of VETORYL Capsules. These included dogs that died or were euthanized because of renal failure, hypoadrenocortical crisis, hemorrhagic diarrhea, and hemorrhagic gastroenteritis.
Foreign Market Experience: The following events were reported voluntarily during post-approval use of VETORYL Capsules in foreign markets. The most serious adverse events were death, adrenal necrosis, hypoadrenocorticism (electrolyte alterations, weakness, collapse, anorexia, lethargy, vomiting, diarrhea, and azotemia), and corticosteroid withdrawal syndrome (weakness, lethargy, anorexia, and weight loss). Additional adverse events included: renal failure, diabetes mellitus, pancreatitis, autoimmune hemolytic anemia, vomiting, diarrhea, anorexia, skin reactions (rash, erythematous skin eruptions), hind limb paresis, seizures, neurological signs from growth of macroadenomas, oral ulceration, and muscle tremors.
POST-APPROVAL EXPERIENCE:
As of June 2013, the following adverse events are based on post-approval adverse drug experience reporting. Not all adverse reactions are reported to FDA CVM. It is not always possible to reliably estimate the adverse event frequency or establish a causal relationship to product exposure using this data. The following adverse events are uled in decreasing order of reporting frequency: anorexia, lethargy/depression, vomiting, diarrhea, elevated liver enzymes, elevated potassium with or without decreased sodium, elevated BUN, decreased Na/K ratio, hypoadrenocorticism, weakness, elevated creatinine, shaking, renal insufficiency. In some cases, death has been reported as an outcome of the adverse events uled above.
To report suspected adverse events and/or obtain a copy of the SDS or for technical assistance, call Dechra Veterinary Products at (866) 933-2472.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at: http://www.fda.gov/reportanimalae
Information For Dog Owners:
Owners should be aware that the most common adverse reactions may include: an unexpected decrease in appetite, vomiting, diarrhea, or lethargy and should receive the Client Information Sheet with the prescription. Owners should be informed that control of hyperadrenocorticism should result in resolution of polyphagia, polyuria and polydipsia. Serious adverse reactions associated with this drug can occur without warning and in some cases result in death (see ADVERSE REACTIONS and POST-APPROVAL EXPERIENCE).
Owners should be advised to discontinue VETORYL Capsules and contact their veterinarian immediately if signs of intolerance such as vomiting, diarrhea, lethargy, poor/reduced appetite, weakness, or collapse are observed. Owners should be advised of the importance of periodic follow-up for all dogs during administration of VETORYL Capsules.
Clinical Pharmacology:
Trilostane absorption is enhanced by administration with food. In healthy dogs, maximal plasma levels of trilostane occur within 1.5 hours, returning to baseline levels within twelve hours, although large inter-dog variation occurs. There is no accumulation of trilostane or its metabolites over time.
Effectiveness:
Eighty-three dogs with hyperadrenocorticism were enrolled in a multi-center US field study. Additionally, 30 dogs with hyperadrenocorticism were enrolled in two UK field studies. Results from these studies demonstrated that treatment with VETORYL Capsules resulted in an improvement in clinical signs (decreased thirst, decreased frequency of urination, decreased panting, and improvement of appetite and activity). Improvement in post-ACTH cortisol levels occurred in most cases within 14 days of starting VETORYL Capsules therapy.
In these three studies, there were a total of 10 dogs diagnosed with hyperadrenocorticism due to an adrenal tumor or due to concurrent pituitary and adrenal tumors. Evaluation of these cases failed to demonstrate a difference in clinical, endocrine, or biochemical response when compared to cases of pituitary-dependent hyperadrenocorticism.
Animal Safety:
In a laboratory study, VETORYL Capsules were administered to 8 healthy 6 month old Beagles per group at 0× (empty capsules), 1×, 3×, and 5× the maximum starting dose of 6.7 mg/kg twice daily for 90 days. Three animals in the 3× group (receiving 20.1 mg/kg twice daily) and five animals in the 5× group (receiving 33.5 mg/kg twice daily) died between Days 23 and 46. They showed one or more of the following clinical signs: decreased appetite, decreased activity, weight loss, dehydration, soft stool, slight muscle tremors, diarrhea, lateral recumbency, and staggering gait. Bloodwork showed hyponatremia, hyperkalemia, and azotemia, consistent with hypoadrenocortical crisis. Post-mortem findings included epithelial necrosis or cystic dilation of duodenal mucosal crypts, gastric mucosal or thymic hemorrhage, atrial thrombosis, pyelitis and cystitis, and inflammation of the lungs.
ACTH stimulated cortisol release was reduced in all dogs treated with VETORYL Capsules. The dogs in the 3× and 5× groups had decreased activity. The 5× dogs had less weight gain than the other groups. The 3× and 5× dogs had lower sodium, albumin, total protein, and cholesterol compared to the control dogs. The 5× dogs had lower mean corpuscular volume than the controls. There was a dose dependent increase in amylase. Post-mortem findings included dose dependent adrenal cortical hypertrophy.
Storage Information:
Store at controlled room temperature 25°C (77°F) with excursions between 15°-30°C (59°-86°F) permitted.
How Supplied:
VETORYL Capsules are available in 5, 10, 20, 30, 60 and 120 mg strengths, packaged in aluminum foil buler cards of 10 capsules, with 3 cards per carton.
VETORYL Capsules 5 mg NDC 17033-105-30 VETORYL Capsules 10 mg NDC 17033-110-30 VETORYL Capsules 20 mg NDC 17033-111-30 VETORYL Capsules 30 mg NDC 17033-130-30 VETORYL Capsules 60 mg NDC 17033-160-30 VETORYL Capsules 120 mg NDC 17033-112-30

Approved by FDA under NADA # 141-291
Manufactured for:Dechra Veterinary Products7015 College Boulevard, Suite 525Overland Park, KS 66211 USA
VETORYL is a registered trademark of Dechra Limited
F2203 Rev. February 2024
Spl Patient Package Insert Section
Dog Owner Information About VETORYL® Capsules (trilostane) Adrenocortical suppressant for oral use in dogs only
VETORYL (pronounced "vet-or-ill") CapsulesGeneric name: trilostane ("try-low-stain")
This summary contains important information about VETORYL Capsules. You should read this information before you start giving your dog VETORYL Capsules and review it each time the prescription is refilled.
This sheet is provided only as a summary and does not take the place of instructions from your veterinarian. Talk to your veterinarian if you do not understand any of this information or if you want to know more about VETORYL Capsules.
What are VETORYL Capsules?
VETORYL Capsules contain an adrenosuppressant drug that is used to treat hyperadrenocorticism in dogs.
VETORYL Capsules are a prescription drug for dogs.
Hyperadrenocorticism (also known as Cushing's disease) is a condition in which excess levels of the hormone cortisol are produced. Cortisol is normally released from the adrenal gland into the bloodstream at times of stress.
In dogs with hyperadrenocorticism, the level of cortisol produced is excessive and, if left untreated, becomes incapacitating.
Characteristic signs are:
- Passing large quantities of urine
- Frequent urination and possible incontinence
- Excessive drinking
- Ravenous appetite
- Lethargy or decreased activity
- Excessive panting
- Pot belly
- Thin skin
- Hair loss or recurrent skin diseases
- Muscle wasting
Your dog may not necessarily display all of these signs.
What should I talk to my veterinarian about before giving VETORYL Capsules?
Periodic follow-up and laboratory testing are important for continual safe use of VETORYL Capsules. Talk with your veterinarian about how often your dog will need to be examined.
Talk to your veterinarian about:
- What tests might be done before VETORYL Capsules are prescribed.
- How often your dog may need to be examined by your veterinarian.
- The risks and benefits of using VETORYL Capsules.
Tell your veterinarian if your dog has ever had the following medical problems:
- Liver disease
- Kidney disease
Tell your veterinarian about:
- Any other medical problems or allergies that your dog has now or has had.
- If your dog is pregnant, nursing or if you plan to breed your dog.
- Any medications your dog is taking, including over-the-counter products and nutritional supplements.
What are the possible side effects that may occur in my dog during therapy?
VETORYL Capsules, like other drugs, may cause some side effects. Serious side effects have been reported in dogs taking VETORYL Capsules. Serious side effects can occur with or without warning and result in death.
Side effects generally involve an over suppression of the adrenal glands (hypoadrenocorticism, also known as Addison's Disease). Look for the following side effects that may indicate your dog is having a problem with VETORYL Capsules or may have another medical problem:
- Depression, lethargy or decrease in activity
- Change in bowel movements (such as diarrhea or loose stools)
- Vomiting
- Stops eating or loses all interest in food
- Weakness and collapse
It is important to stop therapy and contact your veterinarian immediately if you think your dog has a medical problem or side effect from VETORYL Capsule therapy. If you have additional questions about possible side effects, talk to your veterinarian.
As VETORYL Capsules control the hyperadrenocorticism, there should be a decrease in food and water consumption to normal levels. There should also be resolution of excess urination. If, however, there is a dramatic decrease in appetite or your dog stops drinking water, it could be an indication of a side effect requiring treatment.
What kind of results can I expect when my dog is on VETORYL Capsules?
Although VETORYL Capsules ARE NOT A CURE for hyperadrenocorticism, the product can control the clinical signs:
- Response varies from dog to dog.
- Improvement can be seen in most dogs within a few weeks.
- If VETORYL Capsules are discontinued or not given as directed, excess cortisol production can resume and the signs of hyperadrenocorticism can return.
Which dogs should not take VETORYL Capsules?
Your dog should not be given VETORYL Capsules if he/she:
- Has kidney or liver disease.
- Takes certain medications. VETORYL Capsules should be used with caution with several medications used to treat heart disease (some diuretics and ACE inhibitors).
- Is pregnant.
Tell your veterinarian about all medicines you have given your dog in the past, and any medicines that you are planning to give with VETORYL Capsules. This should include other medicines that you can get without a prescription. Your veterinarian may want to check that all of your dog's medicines can be given together.
What should I know about giving VETORYL Capsules to my dog?
- VETORYL Capsules should be given according to your veterinarian's instructions. Your veterinarian will tell you the number of VETORYL Capsules that is right for your dog.
- Administer capsules with food.
- Keep out of reach of children. Not for human use.
- Do not open capsules and do not attempt to split or divide capsules.
- Wash hands after use.
- Do not handle the capsules if pregnant or trying to become pregnant. Studies in laboratory animals have produced birth defects and early pregnancy loss. In the event of accidental ingestion/overdose, seek medical advice immediately and take the labeled container with you.
What do I do in case my dog takes more than the prescribed amount of VETORYL Capsules?
Contact your veterinarian immediately if your dog takes more than the prescribed amount of VETORYL Capsules.
What else should I know about VETORYL Capsules?
This sheet provides a summary of information about VETORYL Capsules. If you have any questions or concerns about VETORYL Capsules or hyperadrenocorticism, talk to your veterinarian.
As with all prescribed medicines, VETORYL Capsules should only be given to the dog for which it was prescribed.
It is important to periodically discuss your dog's response to VETORYL Capsules at regular checkups. Your veterinarian will determine if your dog is responding as expected and if your dog should continue receiving VETORYL Capsules.

Approved by FDA under NADA # 141-291
Manufactured for:Dechra Veterinary Products7015 College Boulevard, Suite 525Overland Park, KS 66211 USA
VETORYL is a registered trademark of Dechra Limited
Rev. February 2024
F2205
Principal Display Panel - 5 Mg Capsule Blister Pack Package
5mg
VETORYL® CAPSULES (trilostane)
Adrenocortical suppressantFor oral use in dogs only
CAUTION: Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
Approved by FDA under NADA # 141-291
30 CapsulesDechra

Principal Display Panel - 20 Mg Capsule Blister Pack Package
20mg
VETORYL® CAPSULES (trilostane)
Adrenocortical suppressantFor oral use in dogs only
CAUTION: Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
Approved by FDA under NADA # 141-291
30 CapsulesDechra

Principal Display Panel - 30 Mg Capsule Blister Pack Package
30mg
VETORYL® CAPSULES (trilostane)
Adrenocortical suppressantFor oral use in dogs only
CAUTION: Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
Approved by FDA under NADA # 141-291
30 CapsulesDechra
Principal Display Panel - 60 Mg Capsule Blister Pack Package
60mg
VETORYL® CAPSULES (trilostane)
Adrenocortical suppressantFor oral use in dogs only
CAUTION: Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
Approved by FDA under NADA # 141-291
30 CapsulesDechra

Principal Display Panel - 10 Mg Capsule Blister Pack Package
10mg
VETORYL® CAPSULES (trilostane)
Adrenocortical suppressantFor oral use in dogs only
CAUTION: Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
Approved by FDA under NADA # 141-291
30 CapsulesDechra

Principal Display Panel - 120 Mg Capsule Blister Pack Package
120mg
VETORYL® CAPSULES (trilostane)
Adrenocortical suppressantFor oral use in dogs only
CAUTION: Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
Approved by FDA under NADA # 141-291
30 CapsulesDechra

DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site


