Vardenafil Hydrochloride (vardenafil hydrochloride 5 mg) Dailymed
Generic: vardenafil hydrochloride is used for the treatment of Impotence, Vasculogenic
IMPRINT: 20
SHAPE: round
COLOR: orange
All Imprints
vardenafil 20 mg oral tablet - 5 round orange
vardenafil 20 mg oral tablet - 10 round orange
vardenafil 20 mg oral tablet - 2 5 round orange
vardenafil 20 mg oral tablet - 20 round orange
Go PRO for all pill images
Recent Major Changes Section
Dosage and Administration ( 2.4 )03/2023
1 Indications And Usage
Vardenafil hydrochloride tablets are indicated for the treatment of erectile dysfunction.
- Vardenafil hydrochloride tablets are a phosphodiesterase 5 (PDE5) inhibitor indicated for the treatment of erectile dysfunction. (
1 )
2 Dosage And Administration
- Vardenafil hydrochloride tablets are taken as needed: For most patients, the starting dose is 10 mg, up to once daily. Increase to 20 mg or decrease to 5 mg based on efficacy/tolerability. (
2.1 )- A starting dose of 5 mg vardenafil hydrochloride tablets should be considered in patients ≥ 65 years of age. (
2.3 )- Vardenafil hydrochloride tablets are taken orally, approximately 60 minutes before sexual activity. (
2.1 )- The maximum recommended dosing frequency is one tablet per day. (
2.1 )- Vardenafil hydrochloride tablets may be taken with or without food. (
2.2 )- If taking strong or moderate inhibitors of CYP3A4, the dose of vardenafil hydrochloride tablets should be adjusted as follows (
2.4 ,5.2 ,7.2 ):
- Ritonavir: No more than 2.5 mg in a 72-hour period
- Cobicistat: No more than 2.5 mg in a 72-hour period
- Indinavir, saquinavir, atazanavir, ketoconazole 400 mg daily, itraconazole 400 mg daily, clarithromycin: No more than 2.5 mg in a 24-hour period
- Ketoconazole 200 mg daily, itraconazole 200 mg daily, erythromycin: No more than 5 mg in a 24-hour period.
- In patients on stable alpha-blocker therapy the recommended starting dose of vardenafil hydrochloride tablets is 5 mg (
2.4 ,5.6 )- The recommended starting dose of vardenafil hydrochloride tablets is 5 mg in patients with moderate hepatic impairment (Child-Pugh B). The maximum dose in patients with moderate hepatic impairment should not exceed 10 mg. (
2.3 ,8.6 )2.1 General Dose Information
For most patients, the recommended starting dose of vardenafil hydrochloride tablets is 10 mg, taken orally, as needed, approximately 60 minutes before sexual activity. The dose may be increased to a maximum recommended dose of 20 mg or decreased to 5 mg based on efficacy and side effects. The maximum recommended dosing frequency is once per day. Sexual stimulation is required for a response to treatment.
2.2 Use with Food
Vardenafil hydrochloride tablets can be taken with or without food.
2.3 Use in Specific Populations
Geriatrics: A starting dose of 5 mg vardenafil hydrochloride tablets should be considered in patients ≥65 years of age [see Use in Specific Populations (8.5)].
Hepatic Impairment: For patients with moderate hepatic impairment (Child-Pugh B), a starting dose of 5 mg vardenafil hydrochloride tablets is recommended. The maximum dose in patients with moderate hepatic impairment should not exceed 10 mg.
Do not use vardenafil hydrochloride tablets in patients with severe hepatic impairment (Child-Pugh C) [ see Warnings and Precautions (5.8), Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)] .
Renal Impairment: Do not use vardenafil hydrochloride tablets in patients on renal dialysis [see Warnings and Precautions (5.9), Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)].
2.4 Concomitant Medications
Nitrates: Concomitant use with nitrates and nitric oxide donors in any form is contraindicated [see Contraindications (4.1)]
Guanylate Cyclase (GC) Stimulators, such as riociguat: Concomitant use is contraindicated [see Contraindications (4.2)].
CYP3A4 Inhibitors: The dosage of vardenafil hydrochloride tablets may require adjustment in patients receiving potent CYP3A4 inhibitors such as ketoconazole, itraconazole, ritonavir, indinavir, saquinavir, atazanavir, cobicistat, and clarithromycin as well as in other patients receiving moderate CYP3A4 inhibitors such as erythromycin [see Drug Interactions (7.2 )]. If taking strong or moderate inhibitors of CYP3A4, the dose of vardenafil hydrochloride tablets should be adjusted as follows:
- Ritonavir: No more than 2.5 mg in a 72-hour period.
- Indinavir, saquinavir, atazanavir, ketoconazole 400 mg daily, itraconazole 400 mg daily, clarithromycin: No more than 2.5 mg in a 24-hour period.
- Ketoconazole 200 mg daily, itraconazole 200 mg daily, erythromycin: No more than 5 mg in a 24-hour period.
- Cobicistat: No more than 2.5 mg in a 72 hour period.
Alpha-Blockers: In those patients who are stable on alpha-blocker therapy, phosphodiesterase type 5 (PDE5) inhibitors should be initiated at the lowest recommended starting dose. Concomitant treatment should be initiated only if the patient is stable on his alpha-blocker therapy. Stepwise increase in alpha-blocker dose may be associated with further lowering of blood pressure in patients taking a phosphodiesterase (PDE5) inhibitor including vardenafil. In those patients who are stable on alpha-blocker therapy, vardenafil hydrochloride tablets should be initiated at a dose of 5 mg (2.5 mg when used concomitantly with certain CYP3A4 inhibitors). [See Warnings and Precautions (5.6) and Drug Interactions (7.1).]
A time interval between dosing should be considered when vardenafil hydrochloride tablets are prescribed concomitantly with alpha-blocker therapy [see Clinical Pharmacology (12.2)] .
3 Dosage Forms And Strengths
Vardenafil hydrochloride tablets are formulated as orange, round, tablets embossed with "2.5", "5", "10" or "20" on one side corresponding to 2.5 mg, 5 mg, 10 mg, and 20 mg of vardenafil, respectively.
- Vardenafil hydrochloride tablets 2.5 mg, 5 mg, 10 mg, 20 mg (
3 )
4 Contraindications
- Administration with nitrates and nitric oxide donors (
2.4 ,4.1 )- Administration with guanylate cyclase (GC) stimulators, such as riociguat (
2.4 ,4.2 )4.1 Nitrates
Administration of vardenafil hydrochloride tablets with nitrates (either regularly and/or intermittently) and nitric oxide donors is contraindicated [see Clinical Pharmacology (12.2)] . Consistent with the effects of PDE5 inhibition on the nitric oxide/cyclic guanosine monophosphate pathway, PDE5 inhibitors, including vardenafil hydrochloride tablets, may potentiate the hypotensive effects of nitrates. A suitable time interval following dosing of vardenafil hydrochloride tablets for the safe administration of nitrates or nitric oxide donors has not been determined.
4.2 Guanylate Cyclase (GC) Stimulators
Do not use vardenafil hydrochloride tablets in patients who are using a GC stimulator, such as riociguat. PDE5 inhibitors, including vardenafil hydrochloride tablets may potentiate the hypotensive effects of GC stimulators.
5 Warnings And Precautions
The evaluation of erectile dysfunction should include a medical assessment, a determination of potential underlying causes and the identification of appropriate treatment.
Before prescribing vardenafil hydrochloride tablets, it is important to note the following:
- Cardiovascular Effects: Patients should not use vardenafil hydrochloride tablets if sex is inadvisable due to cardiovascular status. (
5.1 )- Risk of Priapism: In the event that an erection lasts more than 4 hours, the patient should seek immediate medical assistance. (
5.3 )- Effects on the Eye: Patients should stop use of vardenafil hydrochloride tablets, and seek medical attention in the event of sudden loss of vision in one or both eyes, which could be a sign of nonarteritic anterior ischemic optic neuropathy (NAION). Vardenafil hydrochloride tablets should be used with caution, and only when the anticipated benefits outweigh the risks, in patients with a history of NAION. Patients with a "crowded" optic disc may also be at an increased risk of NAION. (
5.4 ,6.2 )- Sudden Hearing Loss: Patients should stop vardenafil hydrochloride tablets and seek medical attention in the event of sudden decrease or loss in hearing. (
5.5 ,6.2 )- Alpha-Blockers: Caution is advised when PDE5 inhibitors are co-administered with alpha-blockers. In some patients, concomitant use of these two drug classes can lower blood pressure significantly leading to symptomatic hypotension (for example, fainting). (
2.4 ,5.6 )- QT Prolongation: Patients with congenital QT syndrome or taking class IA or III antiarrhythmics should avoid using vardenafil hydrochloride tablets. (
5.7 ,12.2 )- Phenylketonurics: Contains Phenylalanine (
5.13 )5.1 Cardiovascular Effects
General
Physicians should consider the cardiovascular status of their patients, since there is a degree of cardiac risk associated with sexual activity. Therefore, treatment for erectile dysfunction, including vardenafil hydrochloride tablets, should not be used in men for whom sexual activity is not recommended because of their underlying cardiovascular status.
There are no controlled clinical data on the safety or efficacy of vardenafil in the following patients; and therefore its use is not recommended until further information is available: unstable angina; hypotension (resting systolic blood pressure of <90 mmHg); uncontrolled hypertension (>170/110 mmHg); recent history of stroke, life-threatening arrhythmia, or myocardial infarction (within the last 6 months); severe cardiac failure.
Left Ventricular Outflow Obstruction
Patients with left ventricular outflow obstruction, (for example, aortic stenosis and idiopathic hypertrophic subaortic stenosis) can be sensitive to the action of vasodilators including PDE5 inhibitors.
Blood Pressure Effects
Vardenafil hydrochloride tablets have systemic vasodilatory properties that resulted in transient decreases in supine blood pressure in healthy volunteers (mean maximum decrease of 7 mmHg systolic and 8 mmHg diastolic) [see Clinical Pharmacology (12.2)] . While this normally would be expected to be of little consequence in most patients, prior to prescribing vardenafil hydrochloride tablets, physicians should carefully consider whether their patients with underlying cardiovascular disease could be affected adversely by such vasodilatory effects.
5.2 Potential for Drug Interactions with Strong or Moderate CYP3A4 Inhibitors
Concomitant administration with strong CYP3A4 inhibitors (such as ritonavir, indinavir, cobicistat, ketoconazole) or moderate CYP3A4 inhibitors (such as erythromycin) increases plasma concentrations of vardenafil. Dosage adjustment is necessary when vardenafil hydrochloride tablets are administered with certain CYP3A4 inhibitors [see Dosage and Administration (2.4), and Drug Interactions (7.2)].
Long-term safety information is not available on the concomitant administration of vardenafil with HIV protease inhibitors.
5.3 Risk of Priapism
There have been rare reports of prolonged erections greater than 4 hours and priapism (painful erections greater than 6 hours in duration) for this class of compounds, including vardenafil. In the event that an erection persists longer than 4 hours, the patient should seek immediate medical assistance. If priapism is not treated immediately, penile tissue damage and permanent loss of potency may result.
Vardenafil hydrochloride tablets should be used with caution by patients with anatomical deformation of the penis (such as angulation, cavernosal fibrosis, or Peyronie's disease) or by patients who have conditions that may predispose them to priapism (such as sickle cell anemia, multiple myeloma, or leukemia).
5.4 Effects on the Eye
Physicians should advise patients to stop use of all phosphodiesterase type 5 (PDE5) inhibitors, including vardenafil hydrochloride tablets, and seek medical attention in the event of sudden loss of vision in one or both eyes. Such an event may be a sign of non-arteritic anterior ischemic optic neuropathy (NAION), a rare condition and a cause of decreased vision, including permanent loss of vision, that has been reported rarely postmarketing in temporal association with the use of all PDE5 inhibitors. Based on published literature, the annual incidence of NAION is 2.5–11.8 cases per 100,000 in males aged ≥50.
An observational case-crossover study evaluated the risk of NAION when PDE5 inhibitor use, as a class, occurred immediately before NAION onset (within 5 half-lives), compared to PDE5 inhibitor use in a prior time period. The results suggest an approximate 2-fold increase in the risk of NAION, with a risk estimate of 2.15 (95% CI 1.06, 4.34). A similar study reported a consistent result, with a risk estimate of 2.27 (95% CI 0.99, 5.20). Other risk factors for NAION, such as the presence of “crowded” optic disc, may have contributed to the occurrence of NAION in these studies.
Neither the rare postmarketing reports, nor the association of PDE5 inhibitor use and NAION in the observational studies, substantiate a causal relationship between PDE5 inhibitor use and NAIONÂ [see Adverse Reactions (6.2)] .
Physicians should consider whether their patients with underlying NAION risk factors could be adversely affected by use of PDE5 inhibitors. Individuals who have already experienced NAION are at increased risk of NAION recurrence. Therefore, PDE5 inhibitors, including vardenafil hydrochloride tablets, should be used with caution in these patients and only when the anticipated benefits outweigh the risks. Individuals with "crowded" optic disc are also considered at greater risk for NAION compared to the general population, however, evidence is insufficient to support screening of prospective users of PDE5 inhibitors, including vardenafil hydrochloride tablets, for this uncommon condition.
Vardenafil hydrochloride tablets have not been evaluated in patients with known hereditary degenerative retinal disorders, including retinitis pigmentosa, therefore its use is not recommended until further information is available in those patients.
5.5 Sudden Hearing Loss
Physicians should advise patients to stop taking all PDE5 inhibitors, including vardenafil hydrochloride tablets, and seek prompt medical attention in the event of sudden decrease or loss of hearing. These events, which may be accompanied by tinnitus and dizziness, have been reported in temporal association to the intake of PDE5 inhibitors, including vardenafil. It is not possible to determine whether these events are related directly to the use of PDE5 inhibitors or to other factors [see Adverse Reactions (6.2)].
5.6 Alpha-Blockers
Caution is advised when PDE5 inhibitors are co-administered with alpha-blockers. PDE5 inhibitors, including vardenafil hydrochloride tablets, and alpha-adrenergic blocking agents are both vasodilators with blood-pressure lowering effects. When vasodilators are used in combination, an additive effect on blood pressure may be anticipated. In some patients, concomitant use of these two drug classes can lower blood pressure significantly leading to symptomatic hypotension (for example, fainting) [see Drug Interactions (7.1) and Clinical Pharmacology (12.2)] . Consideration should be given to the following:
- Patients should be stable on alpha-blocker therapy prior to initiating a PDE5 inhibitor. Patients who demonstrate hemodynamic instability on alpha-blocker therapy alone are at increased risk of symptomatic hypotension with concomitant use of PDE5 inhibitors.
- In those patients who are stable on alpha-blocker therapy, PDE5 inhibitors should be initiated at the lowest recommended starting dose [see Dosage and Administration (2.4)] .
- In those patients already taking an optimized dose of PDE5 inhibitor, alpha-blocker therapy should be initiated at the lowest dose. Stepwise increase in alpha-blocker dose may be associated with further lowering of blood pressure in patients taking a PDE5 inhibitor.
- Safety of combined use of PDE5 inhibitors and alpha-blockers may be affected by other variables, including intravascular volume depletion and other anti-hypertensive drugs.
5.7 Congenital or Acquired QT Prolongation
In a study of the effect of vardenafil hydrochloride tablets on QT interval in 59 healthy males [see Clinical Pharmacology (12.2)] , therapeutic (10 mg) and supratherapeutic (80 mg) doses of vardenafil and the active control moxifloxacin (400 mg) produced similar increases in QTc interval. A postmarketing study evaluating the effect of combining vardenafil hydrochloride tablets with another drug of comparable QT effect showed an additive QT effect when compared with either drug alone [see Clinical Pharmacology (12.2)] . These observations should be considered in clinical decisions when prescribing vardenafil hydrochloride tablets to patients with known history of QT prolongation or patients who are taking medications known to prolong the QT interval.
Patients taking Class 1A (for example. quinidine, procainamide) or Class III (for example, amiodarone, sotalol) antiarrhythmic medications or those with congenital QT prolongation, should avoid using vardenafil hydrochloride tablets.
5.8 Hepatic Impairment
Dosage adjustment is necessary in patients with moderate hepatic impairment (Child-Pugh B). Do not use vardenafil hydrochloride tablets in patients with severe (Child-Pugh C) hepatic impairment. [See Dosage and Administration (2.3) Clinical Pharmacology (12.3) and Use in Specific Populations (8.6)].
5.9 Renal Impairment
Do not use vardenafil hydrochloride tablets in patients on renal dialysis, as vardenafil has not been evaluated in this population [see Dosage and Administration (2.3) and Use in Specific Populations (8.7)].
5.10 Combination with Other Erectile Dysfunction Therapies
The safety and efficacy of vardenafil hydrochloride tablets used in combination with other treatments for erectile dysfunction have not been studied. Therefore, the use of such combinations is not recommended.
5.11 Effects on Bleeding
In humans, vardenafil alone in doses up to 20 mg does not prolong the bleeding time. There is no clinical evidence of any additive prolongation of the bleeding time when vardenafil is administered with aspirin. Vardenafil hydrochloride tablets have not been administered to patients with bleeding disorders or significant active peptic ulceration. Therefore vardenafil hydrochloride tablets should be administered to these patients after careful benefit-risk assessment.
5.12 Sexually Transmitted Disease
The use of vardenafil hydrochloride tablets offers no protection against sexually transmitted diseases. Counseling of patients about protective measures necessary to guard against sexually transmitted diseases, including the Human Immunodeficiency Virus (HIV), should be considered.
5.13Risks in Patients with Phenylketonuria
Phenylalanine can be harmful to patients with phenylketonuria (PKU). Vardenafil hydrochloride tablets contain phenylalanine, a component of aspartame. Each 2.5 mg vardenafil hydrochloride tablet contains 0.7 mg phenylalanine; each 5 mg tablet contains 1.4 mg phenylalanine; each 10 mg tablet contains 2.8 mg phenylalanine; each 20 mg tablet contains 5.6 mg phenylalanine. Before prescribing vardenafil hydrochloride tablets in a patient with PKU, consider the combined daily amount of phenylalanine from all sources, including vardenafil hydrochloride tablets.
6 Adverse Reactions
The following serious adverse reactions with the use of vardenafil hydrochloride tablets (vardenafil) are discussed elsewhere in the labeling:
- Cardiovascular Effects [see Contraindications (4.1) and Warnings and Precautions (5.1)]
- Priapism [see Warnings and Precautions (5.3)]
- Effects on Eye [see Warnings and Precautions (5.4)]
- Sudden Hearing Loss [see Warnings and Precautions (5.5)]
- QT Prolongation [see Warnings and Precautions (5.7)]
Most common adverse reactions reported ( ≥ 2% of patients) are headache, flushing, nasal congestion, dyspepsia, sinusitis, flu syndrome, dizziness, increased creatine kinase, nausea, back pain. (6.1 )
To report SUSPECTED ADVERSE REACTIONS, contact Lannett Company, Inc. at 1-844-834-0530 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
6.1 Clinical Studies Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Vardenafil hydrochloride tablets were administered to over 4430 men (mean age 56, range 18-89 years; 81% White, 6% Black, 2% Asian, 2% Hispanic and 9% Other) during controlled and uncontrolled clinical trials worldwide. Over 2200 patients were treated for 6 months or longer and 880 patients were treated for at least 1 year.
In placebo-controlled clinical trials, the discontinuation rate due to adverse events was 3.4% for vardenafil hydrochloride tablets compared to 1.1% for placebo.
When vardenafil hydrochloride tablets were taken as recommended in placebo-controlled clinical trials, the following adverse reactions were reported (see Table 1).
Table 1: Adverse Reactions Reported By ≥2% of Patients Treated with Vardenafil Hydrochloride Tablets and More Frequent on Drug than Placebo in Fixed and Flexible Flexible dose studies started all patients at vardenafil hydrochloride tablets 10 mg and allowed decrease in dose to 5 mg or increase in dose to 20 mg based on side effects and efficacy. Dose Randomized, Controlled Trials of 5 mg, 10 mg, or 20 mg VardenafilAdverse Reaction Percentage of Patients Reporting Reactions Placebo N = 1199 Vardenafil Hydrochloride Tablets N = 2203 Headache 4% 15% Flushing 1% 11% Rhinitis 3% 9% Dyspepsia 1% 4% Accidental Injury All the events uled in the above table were deemed to be adverse drug reactions with the exception of accidental injury. 2% 3% Sinusitis 1% 3% Flu Syndrome 2% 3% Dizziness 1% 2% Increased Creatine Kinase 1% 2% Nausea 1% 2%
Back pain was reported in 2.0% of patients treated with vardenafil hydrochloride tablets and 1.7% of patients on placebo.
Placebo-controlled trials suggested a dose effect in the incidence of some adverse reactions (headache, flushing, dyspepsia, nausea, and rhinitis) over the 5 mg, 10 mg, and 20 mg doses of vardenafil hydrochloride tablets.
All Vardenafil Studies: Vardenafil hydrochloride tablets and vardenafil orally disintegrating tablets have been administered to over 17,000 men (mean age 54.5, range 18–89 years; 70% White, 5% Black, 13% Asian, 4% Hispanic and 8% Other) during controlled and uncontrolled clinical trials worldwide. The number of patients treated for 6 months or longer was 3357, and 1350 patients were treated for at least 1 year.
In the placebo-controlled clinical trials for vardenafil hydrochloride tablets and vardenafil orally disintegrating tablets, the discontinuation rate due to adverse events was 1.9% for vardenafil compared to 0.8% for placebo.
The following section identifies additional, less frequent adverse reactions (<2%) reported during the clinical development of vardenafil hydrochloride tablets and vardenafil orally disintegrating tablets. Excluded from this ul are those adverse reactions that are infrequent and minor, those events that may be commonly observed in the absence of drug therapy, and those events that are not reasonably associated with the drug:
Body as a whole: allergic edema and angioedema, feeling unwell, allergic reactions, chest pain
Auditory: tinnitus, vertigo
Cardiovascular: palpitation, tachycardia, angina pectoris, myocardial infarction, ventricular tachyarrhythmias, hypotension
Digestive: nausea, gastrointestinal and abdominal pain, dry mouth, diarrhea, gastroesophageal reflux disease, gastritis, vomiting, increase in transaminases
Musculoskeletal: increase in creatine phosphokinase (CPK), increased muscle tone and cramping, myalgia
Nervous: paresthesia and dysesthesia, somnolence, sleep disorder, syncope, amnesia, seizure
Respiratory: dyspnea, sinus congestion
Skin and appendages: erythema, rash
Ophthalmologic: visual disturbance, ocular hyperemia, visual color distortions, eye pain and eye discomfort, photophobia, increase in intraocular pressure, conjunctivitis
Urogenital: increase in erection, priapism
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of vardenafil hydrochloride tablets. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency or establish a causal relationship to drug exposure.
Ophthalmologic: Non-arteritic anterior ischemic optic neuropathy (NAION), a cause of decreased vision including permanent loss of vision, has been reported rarely postmarketing in temporal association with the use of PDE5 inhibitors, including vardenafil. Most, but not all, of these patients had underlying anatomic or vascular risk factors for development of NAION, including but not necessarily limited to: low cup to disc ratio ("crowded disc"), age over 50, diabetes, hypertension, coronary artery disease, hyperlipidemia and smoking [see Warnings and Precautions (5.4) and Patient Counseling Information (17)] .
Visual disturbances including vision loss (temporary or permanent), such as visual field defect, retinal vein occlusion, and reduced visual acuity, have also been reported rarely in postmarketing experience. It is not possible to determine whether these events are related directly to the use of vardenafil.
Neurologic: Seizure, seizure recurrence and transient global amnesia have been reported postmarketing in temporal association with vardenafil.
Otologic: Cases of sudden decrease or loss of hearing have been reported postmarketing in temporal association with the use of PDE5 inhibitors, including vardenafil. In some cases, medical conditions and other factors were reported that may have also played a role in the otologic adverse events. In many cases, medical follow-up information was limited. It is not possible to determine whether these reported events are related directly to the use of vardenafil, to the patient's underlying risk factors for hearing loss, a combination of these factors, or to other factors [see Patient Counseling Information (17)] .
7 Drug Interactions
- Vardenafil hydrochloride tablets can potentiate the hypotensive effects of nitrates, alpha- blockers, and antihypertensives. (
7.1 )7.1 Potential for Pharmacodynamic Interactions with Vardenafil Hydrochloride Tablets
Nitrates: Concomitant use of vardenafil hydrochloride tablets and nitrates and nitric oxide donors is contraindicated. The blood pressure lowering effects of sublingual nitrates (0.4 mg) taken 1 and 4 hours after vardenafil and increases in heart rate when taken at 1, 4 and 8 hours after vardenafil were potentiated by a 20 mg dose of vardenafil hydrochloride tablets in healthy middle-aged subjects. These effects were not observed when vardenafil hydrochloride tablets 20 mg were taken 24 hours before the nitroglycerin (NTG). Potentiation of the hypotensive effects of nitrates for patients with ischemic heart disease has not been evaluated, and concomitant use of vardenafil hydrochloride tablets and nitrates is contraindicated [see Contraindications (4.1) and Clinical Pharmacology (12.2) .
Alpha-Blockers: Caution is advised when PDE5 inhibitors are co-administered with alpha-blockers. PDE5 inhibitors, including vardenafil hydrochloride tablets and alpha-adrenergic blocking agents are both vasodilators with blood-pressure-lowering effects. When vasodilators are used in combination, an additive effect on blood pressure may be anticipated. Clinical pharmacology studies have been conducted with co-administration of vardenafil with alfuzosin, terazosin or tamsulosin. [See Dosage and Administration (2.4), Warnings and Precautions (5.6), and Clinical Pharmacology (12.2).]
Antihypertensives: Vardenafil hydrochloride tablets may add to the blood pressure lowering effects of antihypertensive agents. In a clinical pharmacology study of patients with erectile dysfunction, single doses of vardenafil 20 mg caused a mean maximum decrease in supine blood pressure of 7 mmHg systolic and 8 mmHg diastolic (compared to placebo), accompanied by a mean maximum increase of heart rate of 4 beats per minute. The maximum decrease in blood pressure occurred between 1 and 4 hours after dosing. Following multiple dosing for 31 days, similar blood pressure responses were observed on Day 31 as on Day 1.
Alcohol: Vardenafil hydrochloride tablets (20 mg) did not potentiate the hypotensive effects of alcohol during the 4-hour observation period in healthy volunteers when administered with alcohol (0.5 g/kg body weight, approximately 40 mL of absolute alcohol in a 70 kg person). Alcohol and vardenafil plasma levels were not altered when dosed simultaneously.
7.2 Effect of Other Drugs on Vardenafil
In vitro studies
Studies in human liver microsomes showed that vardenafil is metabolized primarily by cytochrome P450 (CYP) isoforms 3A4/5, and to a lesser degree by CYP2C9. Therefore, inhibitors of these enzymes are expected to reduce vardenafil clearance [see Dosage and Administration (2.4) and Warnings and Precautions (5.2)] .
In vivo studies
Strong CYP3A4 inhibitors
Ketoconazole (200 mg once daily) produced a 10-fold increase in vardenafil AUC and a 4-fold increase in maximum concentration (C max) when co-administered with vardenafil hydrochloride tablets (5 mg) in healthy volunteers. A 5-mg vardenafil hydrochloride tablet dose should not be exceeded in a 24-hour period when used in combination with 200 mg once daily ketoconazole. Since higher doses of ketoconazole (400 mg daily) may result in higher increases in C max and AUC, a single 2.5 mg dose of vardenafil hydrochloride tablets should not be exceeded in a 24-hour period when used in combination with ketoconazole 400 mg daily. [See Dosage and Administration (2.4) and Warnings and Precautions (5).]
Indinavir (800 mg t.i.d.) co-administered with vardenafil hydrochloride tablets 10 mg resulted in a 16-fold increase in vardenafil AUC, a 7-fold increase in vardenafil C max and a 2-fold increase in vardenafil half-life. It is recommended not to exceed a single 2.5 mg vardenafil hydrochloride tablets dose in a 24-hour period when used in combination with indinavir. [See Dosage and Administration (2.4) and Warnings and Precautions (5.2).]
Ritonavir (600 mg b.i.d.) co-administered with vardenafil hydrochloride tablets 5 mg resulted in a 49-fold increase in vardenafil AUC and a 13Â fold increase in vardenafil C max. The interaction is a consequence of blocking hepatic metabolism of vardenafil by ritonavir, a HIV protease inhibitor and a highly strong CYP3A4 inhibitor, which also inhibits CYP2C9. Ritonavir significantly prolonged the half-life of vardenafil to 26 hours. Consequently, it is recommended not to exceed a single 2.5 mg vardenafil hydrochloride tablets dose in a 72-hour period when used in combination with ritonavir. [See Dosage and Administration (2.4) and Warnings and Precautions (5.2).] .
Cobicistat with vardenafil hydrochloride tablets can result in increased plasma concentrations, therefore it is recommended that a single 2.5 mg dose of vardenafil hydrochloride tablets should not be exceeded in a 72-hour period. [See Dosage and Administration (2.4) and Warnings and Precautions (5).] .
Moderate CYP3A4 inhibitors
Erythromycin (500 mg t.i.d.) produced a 4-fold increase in vardenafil AUC and a 3-fold increase in C max when co administered with vardenafil hydrochloride tablets 5 mg in healthy volunteers. It is recommended not to exceed a single 5 mg dose of vardenafil hydrochloride tablets in a 24-hour period when used in combination with erythromycin. [See Dosage and Administration (2.4) and Warnings and Precautions (5).]
Although specific interactions have not been studied, other CYP3A4 inhibitors, including grapefruit juice would likely increase vardenafil exposure.
Other Drug Interactions
No pharmacokinetic interactions were observed between vardenafil and the following drugs: glyburide, warfarin, digoxin, an antacid based on magnesium-aluminum hydroxide, and ranitidine. In the warfarin study, vardenafil had no effect on the prothrombin time or other pharmacodynamic parameters.
Cimetidine (400 mg b.i.d.) had no effect on vardenafil bioavailability (AUC) and maximum concentration (C max) of vardenafil when co-administered with 20 mg vardenafil hydrochloride tablets in healthy volunteers.
7.3 Effects of Vardenafil on Other Drugs
In vitro studies
Vardenafil and its metabolites had no effect on CYP1A2, 2A6, and 2E1 (Ki >100 micromolar). Weak inhibitory effects toward other isoforms (CYP2C8, 2C9, 2C19, 2D6, 3A4) were found, but Ki values were in excess of plasma concentrations achieved following dosing. The most potent inhibitory activity was observed for vardenafil metabolite M1, which had a Ki of 1.4 micromolar toward CYP3A4, which is about 20 times higher than the M1 C max values after an 80 mg vardenafil dose.
In vitro data suggest that vardenafil has the potential to inhibit P-glycoprotein (P-gp) at therapeutic doses. While concomitant use of vardenafil hydrochloride tablets did not significantly increase plasma concentrations of digoxin, a P-gp substrate, the effect on plasma concentrations of P-gp substrates that are more sensitive than digoxin (e.g. dabigatran) is not known.
In vivo studies
Nifedipine: Vardenafil 20 mg, when co-administered with slow-release nifedipine 30 mg or 60 mg once daily, did not affect the AUC or C max of nifedipine, a drug that is metabolized via CYP3A4. Nifedipine did not alter the plasma levels of vardenafil hydrochloride tablets when taken in combination. In these patients whose hypertension was controlled with nifedipine, vardenafil hydrochloride tablets 20 mg produced mean additional supine systolic/diastolic blood pressure reductions of 6/5 mmHg compared to placebo.
Ritonavir and Indinavir: Upon concomitant administration of 5 mg of vardenafil hydrochloride tablets with 600 mg BID ritonavir, the C max and AUC of ritonavir were reduced by approximately 20%. Upon administration of 10 mg of vardenafil hydrochloride tablets with 800 mg TID indinavir, the C max and AUC of indinavir were reduced by 40% and 30%, respectively.
Aspirin: Vardenafil hydrochloride tablets (10 mg and 20 mg) did not potentiate the increase in bleeding time caused by aspirin (two 81 mg tablets).
Other interactions: Vardenafil hydrochloride tablets had no effect on the pharmacodynamics of glyburide (glucose and insulin concentrations) and warfarin (prothrombin time or other pharmacodynamic parameters).
8 Use In Specific Populations
- Vardenafil hydrochloride tablets are not indicated for use in pediatric patients. (
8.4 )- Do not use vardenafil hydrochloride tablets in patients with severe hepatic impairment (Child- Pugh C). (
8.6 )- Do not use vardenafil hydrochloride tablets in patients on renal dialysis. (
8.7 )8.1 Pregnancy
Risk Summary
Vardenafil hydrochloride tablets are not indicated for use in females.
There are no data with the use of vardenafil hydrochloride tablets in pregnant women to inform any drug-associated risks. In animal reproduction studies conducted in pregnant rats and rabbits, no adverse developmental outcomes were observed with oral administration of vardenafil during organogenesis at exposures for unbound vardenafil and its major metabolite at approximately 100 and 29 times, respectively, the maximum recommended human dose (MRHD) of 20 mg based on AUC (see Data).
Data
Animal Data
No evidence of specific potential for teratogenicity, embryotoxicity or fetotoxicity was observed in rats and rabbits that received vardenafil at up to 18 mg/kg/day during organogenesis. This dose is approximately 100 fold (rat) and 29 fold (rabbit) greater than the AUC values for unbound vardenafil and its major metabolite in humans given the MRHD of 20 mg.
In the rat pre-and postnatal development study, the NOAEL (no observed adverse effect level) for maternal toxicity was 8 mg/kg/day. Retarded physical development of pups in the absence of maternal effects was observed following maternal exposure to 1 and 8 mg/kg possibly due to vasodilatation and/or secretion of the drug into milk. The number of living pups born to rats exposed pre- and postnatally was reduced at 60 mg/kg/day. Based on the results of the pre- and postnatal study, the developmental NOAEL is less than 1 mg/kg/day. Based on plasma exposures in the rat developmental toxicity study, 1 mg/kg/day in the pregnant rat is estimated to produce total AUC values for unbound vardenafil and its major metabolite comparable to the human AUC at the MRHD of 20 mg.
8.2 Lactation
Risk Summary
Vardenafil hydrochloride tablets are not indicated for use in females.
There is no information on the presence of vardenafil and its major metabolite in human milk, the effects on breastfed infant, or the effects on milk production. Vardenafil is present in rat milk of lactating rats (see Data).
Data
Vardenafil was secreted into the milk of lactating rats at concentrations approximately 10-fold greater than found in the plasma. Following a single oral dose of 3 mg/kg, 3.3% of the administered dose was excreted into the milk within 24 hours.
8.4 Pediatric Use
Vardenafil hydrochloride tablets are not indicated for use in pediatric patients. Safety and efficacy have not been established in this population.
8.5 Geriatric Use
Elderly males 65 years of age and older have higher vardenafil plasma concentrations than younger males (18 – 45 years), mean C max and AUC were 34% and 52% higher, respectively. Phase 3 clinical trials included more than 834 elderly patients, and no differences in safety or effectiveness of vardenafil hydrochloride tablets 5, 10, or 20 mg were noted when these elderly patients were compared to younger patients. However, due to increased vardenafil concentrations in the elderly, a starting dose of 5 mg vardenafil hydrochloride tablets should be considered in patients ≥65 years of age [see Clinical Pharmacology (12.3)].
8.6 Hepatic Impairment
Dosage adjustment is necessary in patients with moderate hepatic impairment.
Do not use vardenafil hydrochloride tablets in patients with severe hepatic impairment (Child-Pugh C). Vardenafil has not been evaluated in this patient population.
A starting dose of 5 mg is recommended in patients with moderate hepatic impairment (Child-Pugh B) and the maximum dose should not exceed 10 mg. In volunteers with moderate hepatic impairment, the C max and AUC following a 10 mg vardenafil dose were increased by 130% and 160%, respectively, compared to healthy control subjects. [See Warnings and Precautions (5.8) and Dosage and Administration (2.3).]
In volunteers with mild hepatic impairment (Child-Pugh A), the C max and AUC following a 10 mg vardenafil dose were increased by 22% and 17%, respectively, compared to healthy control subjects. No dosage adjustment is necessary in patients with mild hepatic impairment.
8.7 Renal Impairment
Do not use vardenafil hydrochloride tablets in patients on renal dialysis as vardenafil has not been evaluated in such patients.
No dosage adjustment is necessary in patients with creatinine clearance (CLcr) of 30–80 mL/min. In male volunteers with CLcr = 50-80 ml/min, the pharmacokinetics of vardenafil were similar to those observed in a control group with CLcr >80 mL/min. In male volunteers with CLcr = 30-50 mL/min or CLcr <30 mL/min, the AUC of vardenafil was 20–30% higher compared to that observed in a control group with CLcr >80 mL/min. [See Dosage and Administration (2.3) and Warnings and Precautions (5.9).]
10 Overdosage
The maximum dose of vardenafil hydrochloride tablets for which human data are available is a single 120 mg dose administered to healthy male volunteers. The majority of these subjects experienced reversible back pain/myalgia and/or "abnormal vision." Single doses up to 80 mg vardenafil and multiple doses up to 40 mg vardenafil administered once daily over 4 weeks were tolerated without producing serious adverse side effects.
When 40 mg of vardenafil was administered twice daily, cases of severe back pain were observed. No muscle or neurological toxicity was identified.
In cases of overdose, standard supportive measures should be taken as required. Renal dialysis is not expected to accelerate clearance as vardenafil is highly bound to plasma proteins and not significantly eliminated in the urine.
11 Description
Vardenafil hydrochloride tablets (vardenafil hydrochloride) are administered orally for the treatment of erectile dysfunction. This monohydrochloride salt of vardenafil is a selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type 5 (PDE5).
Vardenafil HCl is designated chemically as piperazine, 1-[[3-(1,4-dihydro-5-methyl-4-oxo-7-propylimidazo[5,1- f][1,2,4]triazin-2-yl)-4-ethoxyphenyl]sulfonyl]-4-ethyl-, monohydrochloride and has the following structural formula:
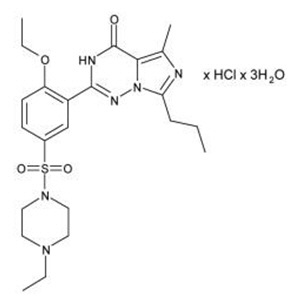
Vardenafil HCl is a nearly colorless, solid substance with a molecular weight of 579.1 g/mol and a solubility of 0.11 mg/mL in water.
Vardenafil hydrochloride tablets are formulated as orange, round, tablets embossed with "2.5", "5", "10" or "20" on one side corresponding to 2.5 mg, 5 mg, 10 mg, and 20 mg of vardenafil, respectively. In addition to the active ingredient, vardenafil HCl, each tablet contains microcrystalline cellulose, crospovidone, colloidal silicon dioxide, magnesium stearate, aspartame, titanium dioxide, ferric oxide red, and ferric oxide yellow.
12 Clinical Pharmacology
12.1 Mechanism of Action
Penile erection is a hemodynamic process initiated by the relaxation of smooth muscle in the corpus cavernosum and its associated arterioles. During sexual stimulation, nitric oxide is released from nerve endings and endothelial cells in the corpus cavernosum. Nitric oxide activates the enzyme guanylate cyclase resulting in increased synthesis of cyclic guanosine monophosphate (cGMP) in the smooth muscle cells of the corpus cavernosum. The cGMP in turn triggers smooth muscle relaxation, allowing increased blood flow into the penis, resulting in erection. The tissue concentration of cGMP is regulated by both the rates of synthesis and degradation via phosphodiesterases (PDEs). The most abundant PDE in the human corpus cavernosum is the cGMP-specific phosphodiesterase type 5 (PDE5); therefore, the inhibition of PDE5 enhances erectile function by increasing the amount of cGMP. Because sexual stimulation is required to initiate the local release of nitric oxide, the inhibition of PDE5 has no effect in the absence of sexual stimulation.
In vitro studies have shown that vardenafil is a selective inhibitor of PDE5. The inhibitory effect of vardenafil is more selective on PDE5 than for other known phosphodiesterases (>15-fold relative to PDE6, >130-fold relative to PDE1, >300-fold relative to PDE11, and >1,000-fold relative to PDE2, 3, 4, 7, 8, 9, and 10).
12.2 Pharmacodynamics
Effects on Blood Pressure
In a clinical pharmacology study of patients with erectile dysfunction, single doses of vardenafil 20 mg caused a mean maximum decrease in supine blood pressure of 7 mmHg systolic and 8 mmHg diastolic (compared to placebo), accompanied by a mean maximum increase of heart rate of 4 beats per minute. The maximum decrease in blood pressure occurred between 1 and 4 hours after dosing. Following multiple dosing for 31 days, similar blood pressure responses were observed on Day 31 as on Day 1. Vardenafil may add to the blood pressure lowering effects of antihypertensive agents [see Drug Interactions (7)] .
Effects on Blood Pressure and Heart Rate when Vardenafil Hydrochloride Tablets are Combined with Nitrates
A study was conducted in which the blood pressure and heart rate response to 0.4 mg nitroglycerin (NTG) sublingually was evaluated in 18 healthy subjects following pretreatment with vardenafil hydrochloride tablets 20 mg at various times before NTG administration. Vardenafil hydrochloride tablets 20 mg caused an additional time-related reduction in blood pressure and increase in heart rate in association with NTG administration. The blood pressure effects were observed when vardenafil hydrochloride tablets 20 mg were dosed 1 or 4 hours before NTG and the heart rate effects were observed when 20 mg was dosed 1, 4, or 8 hours before NTG. Additional blood pressure and heart rate changes were not detected when vardenafil hydrochloride tablets 20 mg were dosed 24 hours before NTG. (See Figure 1)
Figure 1: Placebo-subtracted point estimates (with 90% CI) of mean maximal blood pressure and heart rate effects of pre-dosing with vardenafil 20 mg at 24, 8, 4, and 1 hour before 0.4 mg NTG sublingually
Because the disease state of patients requiring nitrate therapy is anticipated to increase the likelihood of hypotension, the use of vardenafil by patients on nitrate therapy or on nitric oxide donors is contraindicated [see Contraindications (4.1)] .
Blood Pressure Effects in Patients on Stable Alpha-Blocker Treatment
Three clinical pharmacology studies were conducted in patients with benign prostatic hyperplasia (BPH) on stable-dose alpha-blocker treatment, consisting of alfuzosin, tamsulosin or terazosin.
Study 1: This study was designed to evaluate the effect of 5 mg vardenafil compared to placebo when administered to BPH patients on chronic alpha-blocker therapy in two separate cohorts: tamsulosin 0.4 mg daily (cohort 1, n=21) and terazosin 5 or 10 mg daily (cohort 2, n=21). The design was a randomized, double blind, cross-over study with four treatments: vardenafil 5 mg or placebo administered simultaneously with the alpha-blocker and vardenafil 5 mg or placebo administered 6 hours after the alpha-blocker. Blood pressure and pulse were evaluated over the 6-hour interval after vardenafil dosing. For blood pressure (BP) results see Table 2 . One patient after simultaneous treatment with 5 mg vardenafil and 10 mg terazosin exhibited symptomatic hypotension with standing blood pressure of 80/60 mmHg occurring one hour after administration and subsequent mild dizziness and moderate lightheadedness lasting for 6 hours. For vardenafil and placebo, five and two patients, respectively, experienced a decrease in standing systolic blood pressure (SBP) of >30 mmHg following simultaneous administration of terazosin. Hypotension was not observed when vardenafil 5 mg and terazosin were administered 6 hours apart. Following simultaneous administration of vardenafil 5 mg and tamsulosin, two patients had a standing SBP of <85 mmHg. A decrease in standing SBP of >30 mmHg was observed in two patients on tamsulosin receiving simultaneous vardenafil and in one patient receiving simultaneous placebo treatment. When tamsulosin and vardenafil 5 mg were separated by 6 hours, two patients had a standing SBP <85 mmHg and one patient had a decrease in SBP of >30 mmHg. There were no severe adverse events related to hypotension reported during the study. There were no cases of syncope.
Table 2: Mean (95% C.I.) maximal change from baseline in systolic blood pressure (mmHg) following vardenafil 5 mg in BPH patients on stable alpha-blocker therapy (Study 1) Alpha-Blocker Simultaneous dosing of Vardenafil 5 mg and Alpha-Blocker, Placebo-Subtracted Dosing of Vardenafil 5 mg and Alpha-Blocker Separated by 6 Hours, Placebo-Subtracted Terazosin Standing SBP -3 (-6.7, 0.1) -4 (-7.4, -0.5) 5 or 10 mg daily Supine SBP -4 (-6.7, -0.5) -4 (-7.1, -0.7) Tamsulosin Standing SBP -6 (-9.9, -2.1) -4 (-8.3, -0.5) 0.4 mg daily Supine SBP -4 (-7, -0.8) -5 (-7.9, -1.7)
Blood pressure effects (standing SBP) in normotensive men on stable dose of tamsulosin 0.4 mg following simultaneous administration of vardenafil 5 mg or placebo, or following administration of vardenafil 5 mg or placebo separated by 6 hours are shown in Figure 2. Blood pressure effects (standing SBP) in normotensive men on stable dose terazosin (5 or 10 mg) following simultaneous administration of vardenafil 5 mg or placebo, or following administration of vardenafil 5 mg or placebo separated by 6 hours, are shown in Figure 3.
Figure 2: Mean change from baseline in standing systolic blood pressure (mmHg) over 6 hour interval following simultaneous or 6 hr separation administration of vardenafil 5 mg or placebo with stable dose tamsulosin 0.4 mg in normotensive BPH patients (Study 1)
Figure 3: Mean change from baseline in standing systolic blood pressure (mmHg) over 6 hour interval following simultaneous or 6 hr separation administration of vardenafil 5 mg or placebo with stable dose terazosin (5 or 10 mg) in normotensive BPH patients (Study 1)
Study 2: This study was designed to evaluate the effect of 10 mg vardenafil (stage 1) and 20 mg vardenafil (stage 2) compared to placebo, when administered to a single cohort of BPH patients (n=23) on stable therapy with tamsulosin 0.4 mg or 0.8 mg daily for at least four weeks. The design was a randomized, double blind, two-period cross-over study. Vardenafil or placebo was given simultaneously with tamsulosin. Blood pressure and pulse were evaluated over the 6Â hour interval after vardenafil dosing. For BP results see Table 3 . One patient experienced a decrease from baseline in standing SBP of >30 mmHg following vardenafil 10 mg. There were no other instances of outlier blood pressure values (standing SBP <85 mmHg or decrease from baseline in standing SBP of >30 mmHg). Three patients reported dizziness following vardenafil 20 mg. There were no cases of syncope.
Table 3: Mean (95% C.I.) maximal change from baseline in systolic blood pressure (mmHg) following vardenafil 10 and 20 mg in BPH patients on stable alpha-blocker therapy with tamsulosin 0.4 or 0.8 mg daily (Study 2) Vardenafil 10 mg Placebo-subtracted Vardenafil 20 mg Placebo-subtracted Standing SBP -4 (-6.8, -0.3) -4 (-6.8, -1.4) Supine SBP -5 (-8.2, -0.8) -4 (-6.3, -1.8)
Blood pressure effects (standing SBP) in normotensive men on stable dose of tamsulosin 0.4 mg following simultaneous administration of vardenafil 10 mg, vardenafil 20 mg or placebo are shown in Figure 4.
Figure 4: Mean change from baseline in standing systolic blood pressure (mmHg) over 6 hour interval following simultaneous administration of vardenafil 10 mg (Stage 1), vardenafil 20 mg (Stage 2), or placebo with stable dose tamsulosin 0.4 mg in normotensive BPH patients (Study 2)
Study 3: This study was designed to evaluate the effect of single doses of 5 mg vardenafil (stage 1) and 10 mg vardenafil (stage 2) compared to placebo, when administered to a single cohort of BPH patients (n=24) on stable therapy with alfuzosin 10 mg daily for at least four weeks. The design was a randomized, double blind, 3Â period cross-over study. Vardenafil or placebo was administered 4 hours after the administration of alfuzosin. Blood pressure and pulse were evaluated over a 10-hour interval after dosing of vardenafil or placebo. For BP results see Table 4.
Table 4: Mean (95% C.I.) maximal change from baseline in systolic blood pressure (mmHg) following vardenafil 5 and 10 mg in BPH patients on stable alpha-blocker therapy with alfuzosin 10 mg daily (Study 3) Vardenafil 5 mg Placebo-subtracted Vardenafil 10 mg Placebo-subtracted Standing SBP -2 (-5.8, 1.2) -5 (-8.8, -1.6) Supine SBP -1 (-4.1, 2.1) -6 (-9.4, -2.8)
One patient experienced decreases from baseline in standing systolic blood pressure >30 mm Hg after administration of vardenafil 5 mg tablet and vardenafil 10 mg tablet. No instances of standing systolic blood pressure <85 mm Hg were observed during this study. Four patients, one dosed with placebo, two dosed with vardenafil 5 mg tablets and one dosed with vardenafil 10 mg tablets, reported dizziness. Blood pressure effects (standing SBP) in normotensive men on a stable dose of alfuzosin 10 mg following administration of vardenafil 5 mg, vardenafil 10 mg, or placebo separated by 4 hours, are shown in Figure 5.
Figure 5: Mean change from baseline in standing systolic blood pressure (mmHg) over 6 hour interval following 4 hr separation administration of vardenafil 5 mg (stage 1), vardenafil 10 mg (stage 2) or placebo with stable dose alfuzosin 10 mg in BPH patients (Study 3)
Blood pressure effects in normotensive men after forced titration with alpha-blockers:
Two randomized, double blind, placebo-controlled clinical pharmacology studies with healthy normotensive volunteers (age range, 45-74 years) were performed after forced titration of the alpha-blocker terazosin to 10 mg daily over 14 days (n=29), and after initiation of tamsulosin 0.4 mg daily for five days (n=24). There were no severe adverse events related to hypotension in either study. Symptoms of hypotension were a cause for withdrawal in 2 subjects receiving terazosin and in 4 subjects receiving tamsulosin. Instances of outlier blood pressure values (defined as standing SBP <85 mmHg and/or a decrease from baseline of standing SBP >30 mmHg) were observed in 9/24 subjects receiving tamsulosin and 19/29 receiving terazosin. The incidence of subjects with standing SBP <85 mmHg given vardenafil and terazosin to achieve simultaneous T max led to early termination of that arm of the study. In most (7/8) of these subjects, instances of standing SBP <85 mmHg were not associated with symptoms. Among subjects treated with terazosin, outlier values were observed more frequently when vardenafil and terazosin were given to achieve simultaneous T max than when dosing was administered to separate T max by 6 hours. There were 3 cases of dizziness observed with concomitant administration of terazosin and vardenafil. Seven subjects experienced dizziness mainly occurring with simultaneous T max administration of tamsulosin. There were no cases of syncope.
Table 5: Mean (95% C.I.) maximal change in baseline in systolic blood pressure (mmHg) following vardenafil 10 and 20 mg in healthy volunteers on daily alpha-blocker therapy Dosing of Vardenafil and Alpha-Blocker Separated by 6 Hours Simultaneous dosing of Vardenafil and Alpha-Blocker Alpha-Blocker Vardenafil 10 mg Placebo-Subtracted Vardenafil 20 mg Placebo-Subtracted Vardenafil 10 mg Placebo-Subtracted Vardenafil 20 mg Placebo-Subtracted Terazosin 10 mg daily Standing SBP -7 (-10, -3) -11 (-14, -7) -23 (-31, 16) Due to the sample size, confidence intervals may not be an accurate measure for these data. These values represent the range for the difference. -14 (-33, 11) Supine SBP -5 (-8, -2) -7 (-11, -4) -7 (-25, 19) -7 (-31, 22) Tamsulosin 0.4 mg daily Standing SBP -4 (-8, -1) -8 (-11, -4) -8 (-14, -2) -8 (-14, -1) Supine SBP -4 (-8, 0) -7 (-11, -3) -5 (-9, -2) -3 (-7, 0)
Figure 6: Mean change from baseline in standing systolic blood pressure (mmHg) over 6 hour interval following simultaneous or 6 hr separation administration of vardenafil 10 mg, vardenafil 20 mg or placebo with terazosin (10 mg) in healthy volunteers
Figure 7: Mean change from baseline in standing systolic blood pressure (mmHg) over 6 hour interval following simultaneous or 6 hr separation administration of vardenafil 10 mg, vardenafil 20 mg or placebo with tamsulosin (0.4 mg) in healthy volunteers
Effects on Cardiac Electrophysiology
The effect of 10 mg and 80 mg vardenafil on QT interval was evaluated in a single-dose, double-blind, randomized, placebo- and active-controlled (moxifloxacin 400 mg) crossover study in 59 healthy males (81% White, 12% Black, 7% Hispanic) aged 45-60 years. The QT interval was measured at one hour post dose because this time point approximates the average time of peak vardenafil concentration. The 80 mg dose of vardenafil hydrochloride tablets (four times the highest recommended dose) was chosen because this dose yields plasma concentrations covering those observed upon co-administration of a low-dose of vardenafil hydrochloride tablets (5 mg) and 600 mg BID of ritonavir. Of the CYP3A4 inhibitors that have been studied, ritonavir causes the most significant drug-drug interaction with vardenafil. Table 6 summarizes the effect on mean uncorrected QT and mean corrected QT interval (QT c) with different methods of correction (Fridericia and a linear individual correction method) at one hour post-dose. No single correction method is known to be more valid than the other. In this study, the mean increase in heart rate associated with a 10 mg dose of vardenafil hydrochloride tablets compared to placebo was 5 beats/minute and with an 80 mg dose of vardenafil hydrochloride tablets the mean increase was 6 beats/minute.
Table 6. Mean QT and QT c changes in msec (90% CI) from baseline relative to placebo at 1 hour post-dose with different methodologies to correct for the effect of heart rate. Drug/Dose QT Uncorrected (msec) Fridericia QT Correction (msec) Individual QT Correction (msec) Vardenafil 10 mg -2 (-4, 0) 8 (6, 9) 4 (3, 6) Vardenafil 80 mg -2 (-4, 0) 10 (8, 11) 6 (4, 7) Moxifloxacin Active control (drug known to prolong QT) 400 mg3 (1, 5) 8 (6, 9) 7 (5, 8)
Therapeutic and supratherapeutic doses of vardenafil and the active control moxifloxacin produced similar increases in QT c interval. This study, however, was not designed to make direct statistical comparisons between the drug or the dose levels. The clinical impact of these QT c changes is unknown [see Warnings and Precautions (5)] .
In a separate postmarketing study of 44 healthy volunteers, single doses of 10 mg vardenafil hydrochloride tablets resulted in a placebo- subtracted mean change from baseline of QTcF (Fridericia correction) of 5 msec (90% CI: 2,8). Single doses of gatifloxacin 400mg resulted in a placebo-subtracted mean change from baseline QTcF of 4 msec (90% CI: 1,7). When vardenafil hydrochloride tablets 10mg and gatifloxacin 400 mg were co-administered, the mean QTcF change from baseline was additive when compared to either drug alone and produced a mean QTcF change of 9 msec from baseline (90% CI: 6,11). The clinical impact of these QT changes is unknown [see Warnings and Precautions (5.7)] .
Effects on Exercise Treadmill Test in Patients with Coronary Artery Disease (CAD):
In two independent trials that assessed 10 mg (n=41) and 20 mg (n=39) vardenafil, respectively, vardenafil did not alter the total treadmill exercise time compared to placebo. The patient population included men aged 40-80 years with stable exercise-induced angina documented by at least one of the following: 1) prior history of myocardial infarction (MI), coronary artery bypass graft (CABG), percutaneous transluminal coronary angioplasty (PTCA), or stenting (not within 6 months); 2) positive coronary angiogram showing at least 60% narrowing of the diameter of at least one major coronary artery; or 3) a positive stress echocardiogram or stress nuclear perfusion study.
Results of these studies showed that vardenafil hydrochloride tablets did not alter the total treadmill exercise time compared to placebo (10 mg vardenafil hydrochloride tablets vs. placebo: 433±109 and 426±105 seconds, respectively; 20 mg vardenafil hydrochloride tablets vs. placebo: 414±114 and 411±124 seconds, respectively). The total time to angina was not altered by vardenafil hydrochloride tablets when compared to placebo (10 mg vardenafil hydrochloride tablets vs. placebo: 291±123 and 292±110 seconds; 20 mg vardenafil hydrochloride tablets vs. placebo: 354±137 and 347±143 seconds, respectively). The total time to 1 mm or greater ST-segment depression was similar to placebo in both the 10 mg and the 20 mg vardenafil hydrochloride tablet groups (10 mg vardenafil hydrochloride tablets vs. placebo: 380±108 and 334±108 seconds; 20 mg vardenafil hydrochloride tablets vs. placebo: 364±101 and 366±105 seconds, respectively).
Effects on Eye
Single oral doses of phosphodiesterase inhibitors have demonstrated transient dose-related impairment of color discrimination (blue/green) using the Farnsworth-Munsell 100-hue test and reductions in electroretinogram (ERG) b-wave amplitudes, with peak effects near the time of peak plasma levels. These findings are consistent with the inhibition of PDE6 in rods and cones, which is involved in phototransduction in the retina. The findings were most evident one hour after administration, diminishing but still present 6 hours after administration. In a single dose study in 25 normal males, vardenafil hydrochloride tablets 40 mg, twice the maximum daily recommended dose, did not alter visual acuity, intraocular pressure, fundoscopic and slit lamp findings.
In another double-blind, placebo controlled clinical trial, at least 15 doses of 20 mg vardenafil were administered over 8 weeks versus placebo to 52 males. Thirty-two (32) males (62%) of the patients completed the trial. Retinal function was measured by ERG and FM-100 test 2, 6 and 24 hours after dosing. The trial was designed to detect changes in retinal function that might occur in more than 10% of patients. Vardenafil did not produce clinically significant ERG or FM-100 effects in healthy men compared to placebo. Two patients on vardenafil in the trial reported episodes of transient cyanopsia (objects appear blue).
Effects on Sperm Motility/Morphology
There was no effect on sperm motility or morphology after single 20 mg oral doses of vardenafil in healthy volunteers.
12.3 Pharmacokinetics
The pharmacokinetics of vardenafil are approximately dose proportional over the recommended dose range.
Absorption
Mean vardenafil plasma concentrations measured after the administration of a single oral dose of 20 mg to healthy male volunteers are depicted in Figure 8.
Figure 8: Plasma Vardenafil Concentration (Mean ± SD) Curve for a Single 20 mg Vardenafil Hydrochloride Tablets Dose
Vardenafil is rapidly absorbed with absolute bioavailability of approximately 15%. Maximum observed plasma concentrations after a single 20 mg dose in healthy volunteers are usually reached between 30 minutes and 2 hours (median 60 minutes) after oral dosing in the fasted state. Two food-effect studies were conducted which showed that high- fat meals caused a reduction in C max by 18%-50%.
Distribution
The mean steady-state volume of distribution (Vss) for vardenafil is 208 L, indicating extensive tissue distribution. Vardenafil and its major circulating metabolite, M1, are highly bound to plasma proteins (about 95% for parent drug and M1). This protein binding is reversible and independent of total drug concentrations.
Following a single oral dose of 20 mg vardenafil in healthy volunteers, a mean of 0.00018% of the administered dose was obtained in semen 1.5 hours after dosing.
Metabolism
Vardenafil is metabolized predominantly by the hepatic enzyme CYP3A4, with contribution from the CYP3A5 and CYP2C isoforms. The major circulating metabolite, M1, results from desethylation at the piperazine moiety of vardenafil. M1 is subject to further metabolism. The plasma concentration of M1 is approximately 26% that of the parent compound. This metabolite shows a phosphodiesterase selectivity profile similar to that of vardenafil and an in vitro inhibitory potency for PDE5 28% of that of vardenafil. Therefore, M1 accounts for approximately 7% of total pharmacologic activity.
Excretion
The total body clearance of vardenafil is 56 L/h, and the terminal half-life of vardenafil and its primary metabolite (M1) is approximately 4-5 hours. After oral administration, vardenafil is excreted as metabolites predominantly in the feces (approximately 91-95% of administered oral dose) and to a lesser extent in the urine (approximately 2-6% of administered oral dose).
Pharmacokinetics in Specific Populations
Pediatrics
Vardenafil hydrochloride tablets are not indicated for use in pediatric patients. Vardenafil trials were not conducted in the pediatric population.
Geriatric
In a healthy volunteer study of elderly males (≥65 years) and younger males (18–45 years), mean C max and AUC were 34% and 52% higher, respectively, in the elderly males [see Use in Specific Populations (8.5)].
Hepatic Impairment
In volunteers with mild hepatic impairment (Child-Pugh A), the C max and AUC following a 10 mg vardenafil dose were increased by 22% and 17%, respectively, compared to healthy control subjects. In volunteers with moderate hepatic impairment (Child-Pugh B), the C max and AUC following a 10 mg vardenafil dose were increased by 130% and 160%, respectively, compared to healthy control subjects. Vardenafil has not been evaluated in patients with severe (Child-Pugh C) hepatic impairment. [See Dosage and Administration (2.3), Warnings and Precautions (5.8), and Use in Specific Populations (8.6).]
Renal Impairment
In male volunteers with CLcr = 50–80 mL/min, the pharmacokinetics of vardenafil were similar to those observed in a control group with CLcr >80 mL/min. In male volunteers with CLcr = 30–50 mL/min or CLcr <30 mL/min renal impairment groups, the AUC of vardenafil was 20–30% higher compared to that observed in a control group with CLcr >80 mL/min). Vardenafil pharmacokinetics have not been evaluated in patients requiring renal dialysis. [See Dosage and Administration (2.3), Warnings and Precautions (5.9), and Use in Specific Populations (8.7).]
13 Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY SECTION
Carcinogenesis
Vardenafil was not carcinogenic in rats and mice when administered daily for 24 months. In these studies systemic drug exposures (AUCs) for unbound (free) vardenafil and its major metabolite were approximately 400- and 170-fold for male and female rats, respectively, and 21- and 37-fold for male and female mice, respectively, the exposures observed in human males given the Maximum Recommended Human Dose (MRHD) of 20 mg.
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY SECTION
Mutagenesis
Vardenafil was not mutagenic as assessed in either the in vitro bacterial Ames assay or the forward mutation assay in Chinese hamster V 79 cells. Vardenafil was not clastogenic as assessed in either the in vitro chromosomal aberration test or the in vivo mouse micronucleus test.
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY SECTION
Impairment of Fertility
Vardenafil did not impair fertility in male and female rats administered doses up to 100 mg/kg/day for 28 days prior to mating in male, and for 14 days prior to mating and through day 7 of gestation in females. In a corresponding 1-month rat toxicity study, this dose produced an AUC value for unbound vardenafil 200 fold greater than AUC in humans at the MRHD of 20 mg.
14 Clinical Studies
Vardenafil hydrochloride tablets were evaluated in four major double-blind, randomized, placebo-controlled, fixed-dose, parallel design, multicenter trials in 2431 men aged 20-83 (mean age 57 years; 78% White, 7% Black, 2% Asian, 3% Hispanic and 10% Other/Unknown). The doses of vardenafil hydrochloride tablets in these studies were 5 mg, 10 mg, and 20 mg. Two of these trials were conducted in the general erectile dysfunction (ED) population and two in special ED populations (one in patients with diabetes mellitus and one in post-prostatectomy patients). Vardenafil hydrochloride tablets were dosed without regard to meals on an as needed basis in men with ED, many of whom had multiple other medical conditions. The primary endpoints were assessed at 3 months.
Primary efficacy assessment in all four major trials was by means of the Erectile Function (EF) Domain score of the validated International Index of Erectile Function (IIEF) Questionnaire and two questions from the Sexual Encounter Profile (SEP) dealing with the ability to achieve vaginal penetration (SEP2), and the ability to maintain an erection long enough for successful intercourse (SEP3).
In all four fixed-dose efficacy trials, vardenafil hydrochloride tablets showed clinically meaningful and statistically significant improvement in the EF Domain, SEP2, and SEP3 scores compared to placebo. The mean baseline EF Domain score in these trials was 11.8 (scores range from 0-30 where lower scores represent more severe disease). Vardenafil hydrochloride tablets (5 mg, 10 mg, and 20 mg) were effective in all age categories (<45, 45 to <65, and ≥65 years) and was also effective regardless of race (White, Black, Other).
14.1 Trials in a General Erectile Dysfunction Population
In the major North American fixed-dose trial, 762 patients (mean age 57, range 20-83 years; 79% White, 13% Black, 4% Hispanic, 2% Asian and 2% Other) were evaluated. The mean baseline EF Domain scores were 13, 13, 13, 14 for the vardenafil hydrochloride tablets 5 mg, 10 mg, 20 mg and placebo groups, respectively. There was significant improvement (p <0.0001) at 3 months with vardenafil hydrochloride tablets (EF Domain scores of 18, 21, 21, for the 5 mg, 10 mg, and 20 mg dose groups, respectively) compared to the placebo group (EF Domain score of 15). The European trial (total N=803) confirmed these results. The improvement in mean score was maintained at all doses at 6 months in the North American trial.
In the North American trial, vardenafil hydrochloride tablets significantly improved the rates of achieving an erection sufficient for penetration (SEP2) at doses of 5 mg, 10 mg, and 20 mg compared to placebo (65%, 75%, and 80%, respectively, compared to a 52% response in the placebo group at 3 months; p <0.0001). The European trial confirmed these results.
Vardenafil hydrochloride tablets demonstrated a clinically meaningful and statistically significant increase in the overall per-patient rate of maintenance of erection to successful intercourse (SEP3) (51% on 5 mg, 64% on 10 mg, and 65% on 20 mg, respectively, compared to 32% on placebo; p <0.0001) at 3 months in the North American trial. The European trial showed comparable efficacy. This improvement in mean score was maintained at all doses at 6 months in the North American trial.
14.2 Trial in Patients with ED and Diabetes Mellitus
Vardenafil hydrochloride tablets demonstrated clinically meaningful and statistically significant improvement in erectile function in a prospective, fixed-dose (10 and 20 mg vardenafil hydrochloride tablets), double-blind, placebo-controlled trial of patients with diabetes mellitus (n=439; mean age 57 years, range 33-81; 80% White, 9% Black, 8% Hispanic, and 3% Other).
Significant improvements in the EF Domain were shown in this study (EF Domain scores of 17 on 10 mg vardenafil hydrochloride tablets and 19 on 20 mg vardenafil hydrochloride tablets compared to 13 on placebo; p <0.0001).
Vardenafil hydrochloride tablets significantly improved the overall per-patient rate of achieving an erection sufficient for penetration (SEP2) (61% on 10 mg and 64% on 20 mg vardenafil hydrochloride tablets compared to 36% on placebo; p <0.0001).
Vardenafil hydrochloride tablets demonstrated a clinically meaningful and statistically significant increase in the overall per-patient rate of maintenance of erection to successful intercourse (SEP3) (49% on 10 mg, 54% on 20 mg vardenafil hydrochloride tablets compared to 23% on placebo; p <0.0001).
14.3 Trial in Patients with ED after Radical Prostatectomy
Vardenafil hydrochloride tablets demonstrated clinically meaningful and statistically significant improvement in erectile function in a prospective, fixed-dose (10 and 20 mg vardenafil hydrochloride tablets), double-blind, placebo-controlled trial in post-prostatectomy patients (n=427, mean age 60, range 44-77 years; 93% White, 5% Black, 2% Other).
Significant improvements in the EF Domain were shown in this study (EF Domain scores of 15 on 10 mg vardenafil hydrochloride tablets and 15 on 20 mg vardenafil hydrochloride tablets compared to 9 on placebo; p <0.0001).
Vardenafil hydrochloride tablets significantly improved the overall per-patient rate of achieving an erection sufficient for penetration (SEP2) (47% on 10 mg and 48% on 20 mg vardenafil hydrochloride tablets compared to 22% on placebo; p <0.0001).
Vardenafil hydrochloride tablets demonstrated a clinically meaningful and statistically significant increase in the overall per-patient rate of maintenance of erection to successful intercourse (SEP3) (37% on 10 mg, 34% on 20 mg vardenafil hydrochloride tablets compared to 10% on placebo; p <0.0001).
16 How Supplied/storage And Handling
Vardenafil hydrochloride tablets are formulated as orange, round, tablets embossed with "2.5", "5", "10" or "20" on one side equivalent to 2.5 mg, 5 mg, 10 mg, and 20 mg of vardenafil, respectively
Package Strength NDC Code Bottles of 30 2.5 mg 0527-2800-32 5 mg 0527-2801-32 10 mg 0527-2802-32 20 mg 0527-2803-32 STORAGE AND HANDLING SECTION
Store at 25°C (77°F); excursions permitted within15-30°C (59-86°F) [see USP Controlled Room Temperature].
17 Patient Counseling Information
"See FDA-approved patient labeling (Patient Information)"
Nitrates
Inform patients that vardenafil hydrochloride tablets are contraindicated with regular and/or intermittent use of organic nitrates. Patients should be counseled that concomitant use of vardenafil hydrochloride tablets with nitrates could cause blood pressure to suddenly drop to an unsafe level, resulting in dizziness, syncope, or even heart attack or stroke.
Guanylate Cyclase (GC) Stimulators
Inform patients that vardenafil hydrochloride tablets are contraindicated in patients who use guanylate cyclase stimulators, such as riociguat.
Cardiovascular
Discuss with patients the potential cardiac risk of sexual activity for patients with preexisting cardiovascular risk factors.
Concomitant Use with Drugs which Lower Blood Pressure
Inform patients that in some patients concomitant use of PDE5 inhibitors, including vardenafil hydrochloride tablets, with alpha-blockers can lower blood pressure significantly leading to symptomatic hypotension (for example, fainting).
Patients prescribed vardenafil hydrochloride tablets who are taking alpha-blockers should be started on the lowest recommended starting dose of vardenafil hydrochloride tablets [see Dosage and Administration (2.4) and Drug Interactions (7)]. Patients should be advised of the possible occurrence of symptoms related to postural hypotension and appropriate countermeasures. Patients should be advised to contact the prescribing physician if other anti-hypertensive drugs or new medications that may interact with vardenafil hydrochloride tablets are prescribed by another healthcare provider.
Recommended Administration
Discuss with patients the appropriate use of vardenafil hydrochloride tablets and its anticipated benefits. It should be explained that sexual stimulation is required for an erection to occur after taking vardenafil hydrochloride tablets. Vardenafil hydrochloride tablets should be taken approximately 60 minutes before sexual activity. Patients should be counseled regarding the dosing of vardenafil hydrochloride tablets especially regarding the maximum daily dose. Patients should be advised to contact their healthcare provider for dose modification if they are not satisfied with the quality of their sexual performance with vardenafil hydrochloride tablets or in the case of an unwanted effect.
Priapism
Inform patients that there have been rare reports of prolonged erections greater than 4 hours and priapism (painful erections greater than 6 hours in duration) for vardenafil hydrochloride tablets and this class of compounds. In the event that an erection persists longer than 4 hours, the patient should seek immediate medical assistance. If priapism is not treated immediately, penile tissue damage and permanent loss of potency may result.
Drug Interactions
Advise patients to contact the prescribing physician if new medications that may interact with vardenafil hydrochloride tablets are prescribed by another healthcare provider.
Sudden Loss of Vision
Inform patients to stop use of all PDE5 inhibitors, including vardenafil hydrochloride tablets, and seek medical attention in the event of sudden loss of vision in one or both eyes. Such an event may be a sign of non-arteritic anterior ischemic optic neuropathy (NAION), a cause of decreased vision, including permanent loss of vision, that has been reported rarely post-marketing in temporal association with the use of all PDE5 inhibitors. Physicians should also discuss with patients the increased risk of NAION in individuals who have already experienced NAION in one eye. Physicians should also discuss with patients the increased risk of NAION among the general population in patients with a "crowded" optic disc, although evidence is insufficient to support screening of prospective users of PDE5 inhibitor, including vardenafil hydrochloride tablets, for this uncommon condition [see Warnings and Precautions (5.4) and Adverse Reactions (6.1)] .
Sudden Hearing Loss
Advise patients to stop taking PDE5 inhibitors, including vardenafil hydrochloride tablets, and seek prompt medical attention in the event of sudden decrease or loss of hearing. These events, which may be accompanied by tinnitus and dizziness, have been reported in temporal association to the intake of PDE5 inhibitors, including vardenafil hydrochloride tablets. It is not possible to determine whether these events are related directly to the use of PDE5 inhibitors or to other factors [see Adverse Reactions (6)].
Sexually Transmitted Disease
Inform patients that vardenafil hydrochloride tablets offer no protection against sexually transmitted diseases. Counsel patients that protective measures necessary to guard against sexually transmitted diseases, including the Human Immunodeficiency Virus (HIV), should be considered.
Dose Adjustment
Inform patients that the recommended starting dose of vardenafil hydrochloride tablets is 10 mg. The dose may be increased to a maximum recommended dose of 20 mg or decreased to 5 mg based on efficacy and tolerability. The maximum recommended dosing frequency is one tablet per day.
Spl Patient Package Insert Section
FDA-approved patient labeling
VARDENAFIL HYDROCHLORIDE (var-DEN-a-fil HYE-droe-KLOR-ide) TABLETS
Read the Patient Information about vardenafil hydrochloride tablets before you start taking them and again each time you get a refill. There may be new information. You may also find it helpful to share this information with your partner. This leaflet does not take the place of talking with your doctor. You and your doctor should talk about vardenafil hydrochloride tablets when you start taking it and at regular checkups. If you do not understand the information, or have questions, talk with your doctor or pharmacist.
WHAT IMPORTANT INFORMATION SHOULD YOU KNOW ABOUT VARDENAFIL HYDROCHLORIDE TABLETS?
Vardenafil hydrochloride tablets can cause your blood pressure to drop suddenly to an unsafe level if it is taken with certain other medicines. With a sudden drop in blood pressure, you could get dizzy, faint, or have a heart attack or stroke.
Do not take vardenafil hydrochloride tablets if you:
- Take any medicines called "nitrates" (often used to control chest pain, also known as angina).
- Use recreational drugs called "poppers" like amyl nitrate and butyl nitrate.
- Take riociguat (Adempas ®), a guanylate cyclase stimulator, a medicine that treats pulmonary arterial hypertension and chronic-thromboembolic pulmonary hypertension.
(See " Who Should Not Take Vardenafil Hydrochloride Tablets?")
Tell all your healthcare providers that you take vardenafil hydrochloride tablets. If you need emergency medical care for a heart problem, it will be important for your healthcare provider to know when you last took vardenafil hydrochloride tablets.
WHAT ARE VARDENAFIL HYDROCHLORIDE TABLETS?
Vardenafil hydrochloride tablets are a prescription medicine taken by mouth for the treatment of erectile dysfunction (ED) in men.
ED is a condition where the penis does not harden and expand when a man is sexually excited, or when he cannot keep an erection. A man who has trouble getting or keeping an erection should see his doctor for help if the condition bothers him. Vardenafil hydrochloride tablets may help a man with ED get and keep an erection when he is sexually excited.
Vardenafil hydrochloride tablets do not:
- Cure ED
- Increase a man's sexual desire
- Protect a man or his partner from sexually transmitted diseases, including HIV. Speak to your doctor about ways to guard against sexually transmitted diseases.
- Serve as a male form of birth control.
Vardenafil hydrochloride tablets are only for men with ED. Vardenafil hydrochloride tablets are not for women or children. Vardenafil hydrochloride tablets must be used only under a doctor's care.
HOW DO VARDENAFIL HYDROCHLORIDE TABLETS WORK?
When a man is sexually stimulated, his body's normal physical response is to increase blood flow to his penis. This results in an erection. Vardenafil hydrochloride tablets help increase blood flow to the penis and may help men with ED get and keep an erection satisfactory for sexual activity. Once a man has completed sexual activity, blood flow to his penis decreases, and his erection goes away.
WHO CAN TAKE VARDENAFIL HYDROCHLORIDE TABLETS?
Talk to your doctor to decide if vardenafil hydrochloride tablets are right for you.
Vardenafil hydrochloride tablets have been shown to be effective in men over the age of 18 years who have erectile dysfunction, including men with diabetes or who have undergone prostatectomy.
WHO SHOULD NOT TAKE VARDENAFIL HYDROCHLORIDE TABLETS?
Do not take vardenafil hydrochloride tablets if you:
- Take any medicines called "nitrates" (See " What important information should you know about vardenafil hydrochloride tablets? "). Nitrates are commonly used to treat angina. Angina is a symptom of heart disease and can cause pain in your chest, jaw, or down your arm. Medicines called nitrates include nitroglycerin that is found in tablets, sprays, ointments, pastes, or patches. Nitrates can also be found in other medicines such as isosorbide dinitrate or isosorbide mononitrate. Some recreational drugs called "poppers" also contain nitrates, such as amyl nitrate and butyl nitrate. Do not use vardenafil hydrochloride tablets if you are using these drugs. Ask your doctor or pharmacist if you are not sure if any of your medicines are nitrates.
- Take riociguat, a guanylate cyclase stimulator, a medicine that treats pulmonary arterial hypertension and chronic-throembolic pulmonary hypertension.
- Have been told by your healthcare provider to not have sexual activity because of health problems. Sexual activity can put an extra strain on your heart, especially if your heart is already weak from a heart attack or heart disease.
WHAT SHOULD YOU DISCUSS WITH YOUR DOCTOR BEFORE TAKING VARDENAFIL HYDROCHLORIDE TABLETS?
Before taking vardenafil hydrochloride tablets, tell your doctor about all your medical problems, including if you:
- Have heart problems such as angina, heart failure, irregular heartbeats, or have had a heart attack. Ask your doctor if it is safe for you to have sexual activity.
- Have low blood pressure or have high blood pressure that is not controlled.
- Have pulmonary hypertension.
- Have had a stroke.
- Have had a seizure.
- Or any family members have a rare heart condition known as prolongation of the QT interval (long QT syndrome).
- Have liver problems.
- Have kidney problems and require dialysis.
- Have retinitis pigmentosa, a rare genetic (runs in families) eye disease
- Have ever had severe vision loss, or if you have an eye condition called non-arteritic anterior ischemic optic neuropathy (NAION).
- Have stomach ulcers.
- Have a bleeding problem.
- Have a deformed penis shape or Peyronie's disease.
- Have had an erection that lasted more than 4 hours.
- Have blood cell problems such as sickle cell anemia, multiple myeloma, or leukemia.
- Have hearing problems.
CAN OTHER MEDICATIONS AFFECT VARDENAFIL HYDROCHLORIDE TABLETS?
Tell your doctor about all the medicines you take including prescription and non-prescription medicines, vitamins, and herbal supplements. Vardenafil hydrochloride tablets and other medicines may affect each other. Always check with your doctor before starting or stopping any medicines. Especially tell your doctor if you take any of the following:
- Medicines called nitrates (see " What important information should you know about vardenafil hydrochloride tablets?").
- Ketoconazole or itraconazole (such as Nizoral® or Sporanox®).
- Ritonavir (Norvir®) or indinavir sulfate (Crixivan®) saquinavir (Fortavase® or Invirase®) or atazanavir (Reyataz®) or cobicistat.
- Erythromycin or clarithromycin.
- Medicines called alpha-blockers. These include Hytrin® (terazosin HCl), Flomax® (tamsulosin HCl), Cardura® (doxazosin mesylate), Minipress® (prazosin HCl), Rapaflo® (silodosin) or Uroxatral® (alfuzosin HCl). Alpha-blockers are sometimes prescribed for prostate problems or high blood pressure. In some patients the use of PDE5 inhibitor drugs, including vardenafil hydrochloride tablets, with alpha-blockers can lower blood pressure significantly leading to fainting.
- You should contact the prescribing physician if alpha-blockers or other drugs that lower blood pressure are prescribed by another healthcare provider.
- Medicines that treat abnormal heartbeat. These include quinidine, procainamide, amiodarone and sotalol.
- Other medicines or treatments for ED.
HOW SHOULD YOU TAKE VARDENAFIL HYDROCHLORIDE TABLETS?
Take vardenafil hydrochloride tablets exactly as your doctor prescribes. Do not take more than one vardenafil hydrochloride tablet a day. Doses should be taken at least 24 hours apart. Some men can only take a low dose of vardenafil hydrochloride tablets because of medical conditions or medicines they take. Your doctor will prescribe the dose that is right for you.
- If you are older than 65 or have liver problems, your doctor may start you on a lower dose of vardenafil hydrochloride tablets.
- If you have prostate problems or high blood pressure, for which you take medicines called alpha-blockers, your doctor may start you on a lower dose of vardenafil hydrochloride tablets.
- If you are taking certain other medicines your doctor may prescribe a lower starting dose and limit you to one dose of vardenafil hydrochloride tablets in a 72-hour (3 days) period.
Take 1 vardenafil hydrochloride tablet about 1 hour (60 minutes) before sexual activity. Some form of sexual stimulation is needed for an erection to happen with vardenafil hydrochloride tablet. Vardenafil hydrochloride tablets may be taken with or without meals.
Do not change your dose of vardenafil hydrochloride tablets without talking to your doctor. Your doctor may lower your dose or raise your dose, depending on how your body reacts to vardenafil hydrochloride tablets.
Call your doctor or emergency room immediately if you accidentally took more vardenafil hydrochloride tablets than prescribed.
WHAT ARE THE POSSIBLE SIDE EFFECTS OF VARDENAFIL HYDROCHLORIDE TABLETS?
The most common side effects with vardenafil hydrochloride tablets are headache, flushing, stuffy or runny nose, indigestion, upset stomach, dizziness or back pain. These side effects usually go away after a few hours. Call your doctor if you get a side effect that bothers you or one that will not go away.
Vardenafil hydrochloride tablets may uncommonly cause:
- An erection that won't go away (priapism). If you get an erection that lasts more than 4 hours, get medical help right away. Priapism must be treated as soon as possible or lasting damage can happen to your penis including the inability to have erections.
- Color vision changes, such as seeing a blue tinge to objects or having difficulty telling the difference between the colors blue and green.
In rare instances, men taking PDE5 inhibitors (oral erectile dysfunction medicines, including vardenafil hydrochloride tablets) reported a sudden decrease or loss of vision in one or both eyes. It is uncertain whether PDE5 inhibitors directly cause the vision loss. If you experience sudden decrease or loss of vision, stop taking PDE5 inhibitors, including vardenafil hydrochloride tablets, and call a doctor right away.
Sudden loss or decrease in hearing, sometimes with ringing in the ears and dizziness, has been rarely reported in people taking PDE5 inhibitors, including vardenafil hydrochloride tablets. It is not possible to determine whether these events are related directly to the PDE5 inhibitors, to other diseases or medications, to other factors, or to a combination of factors. If you experience these symptoms, stop taking vardenafil hydrochloride tablets and contact a doctor right away.
These are not all the side effects of vardenafil hydrochloride tablets. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1Â-800-FDA-1088.
HOW SHOULD VARDENAFIL HYDROCHLORIDE TABLETS BE STORED?
- Store vardenafil hydrochloride tablets at room temperature between 59–86° F (15–30° C).
- Keep vardenafil hydrochloride tablets and all medicines out of the reach of children.
GENERAL INFORMATION ABOUT VARDENAFIL HYDROCHLORIDE TABLETS
Medicines are sometimes prescribed for conditions other than those described in patient information leaflets. Do not use vardenafil hydrochloride tablets for a condition for which it was not prescribed. Do not give vardenafil hydrochloride tablets to other people, even if they have the same symptoms that you have. It may harm them.
This leaflet summarizes the most important information about vardenafil hydrochloride tablets. If you would like more information, talk with your healthcare provider. You can ask your doctor or pharmacist for information about vardenafil hydrochloride tablets that is written for health professionals.
WHAT ARE THE INGREDIENTS OF VARDENAFIL HYDROCHLORIDE TABLETS?
Active Ingredient: vardenafil hydrochloride
Inactive Ingredients: microcrystalline cellulose, crospovidone, colloidal silicon dioxide, magnesium stearate, aspartame, titanium dioxide, ferric oxide red, and ferric oxide yellow.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Products cited in vardenafil hydrochloride tablets USPI Norvir (ritonavir) is a trademark of Abbott Laboratories Crixivan (indinavir sulfate) is a trademark of Merck & Co., Inc. Invirase or Fortavase (saquinavir mesylate) is a trademark of Roche Laboratories Inc. Reyataz (atazanavir sulfate) is a trademark of Bristol-Myers Squibb Company Nizoral (ketoconazole) is a trademark of Johnson & Johnson Sporanox (itraconazole) is a trademark of Johnson & Johnson Hytrin (terazosin HCl) is a trademark of Abbott Laboratories Flomax (tamsulosin HCl) is a trademark of Yamanouchi Pharmaceutical Co., Ltd. Cardura (doxazosin mesylate) is a trademark of Pfizer Inc. Minipress (prazosin HCl) is a trademark of Pfizer Inc. Rapaflo (silodosin) is a trademark of Watson Pharma Inc. Uroxatral (alfuzosin HCl) is a trademark of Sanofi-Synthelabo
Manufactured by: Rivopharm SA 6928, Manno, Switzerland
Distributed by: Lannett Company, Inc. Philadelphia, PA 19136
Rx Only
L6897B Rev. 09/2023
Principal Display Panel - 2.5 Mg Tablet Bottle Label
NDC 0527-2800-32
Vardenafil Hydrochloride Tablets
2.5 mg
Rx only 30 Tablets

Principal Display Panel - 5 Mg Tablet Bottle Label
NDC 0527-2801-32
Vardenafil Hydrochloride Tablets
5 mg
Rx only 30 Tablets

Principal Display Panel - 10 Mg Tablet Bottle Label
NDC 0527-2802-32
Vardenafil Hydrochloride Tablets
10 mg
Rx only 30 Tablets

Principal Display Panel - 20 Mg Tablet Bottle Label
NDC 0527-2803-32
Vardenafil Hydrochloride Tablets
20 mg
Rx only 30 Tablets

DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site


