Phytonadione (phytonadione 5 mg) Dailymed
Generic: phytonadione is used for the treatment of Abnormalities, Drug-Induced Hypoprothrombinemias Infant, Premature, Diseases
Go PRO for all pill images
1 Indications And Usage
Phytonadione tablets are indicated for the treatment of adults with the following coagulation disorders which are due to faulty formation of factors II, VII, IX and X when caused by vitamin K deficiency or interference with vitamin K activity.
- Anticoagulant-induced hypoprothrombinemia caused by coumarin or indanedione derivatives.
- Hypoprothrombinemia secondary to antibacterial therapy.
- Hypoprothrombinemia secondary to factors limiting absorpsion or synthesis of vitamin K, e.g., obstructive jaundice, biliary fistula, sprue, ulcerative colitis, celiac disease, intestinal resection, cystic fibrosis of the pancrease, and regional enteritis.
- Other drug-induced hypoprothrombinemia where it is definitely shown that the result is due to interference with vitamin K metabolism, e.g., salicylates.
Phytonadione is a vitamin K replacement indicated for the treatment of adults with the following coagulation disorders which are due to faulty formation of factors II, VII, IX and X when caused by vitamin K deficiency or interference with vitamin K activity:
- Anticoagulant-induced prothrombin deficiency caused by coumarin or indanedione derivatives.
(1) - Hypoprothrombinemia secondary to antibacterial therapy.
(1) - Hypoprothrombinemia secondary to factors limiting absorption or synthesis of vitamin K, e.g., obstructive jaundice, biliary fistula, sprue, ulcerative colitis, celiac disease, intestinal resection, cystic fibrosis of the pancreas, and regional enteritis.
(1) - Other drug-induced hypoprothrombinemia where it is definitively shown that the result is due to interference with vitamin K metabolism, e.g., salicylates.
(1)
2 Dosage And Administration
- Anticoagulant-Induced Prothrombin Deficiency: 2.5 mg to 10 mg or up to 25 mg.
(2.2) - Hypoprothrombinemia Due to Other Causes: 2.5 mg to 25 mg or more.
(2.2) - Must be given with bile salts when endogenous supply of bile to gastrointestinal track is deficient.
(2.1) 2.1 Dosing Considerations
Avoid the oral route when the clinical disorder would prevent proper absorption. Bile salts must be given with the tablets when the endogenous supply of bile to the gastrointestinal tract is deficient. The coagulant effects of phytonadione tablets are not immediate; improvement of international normalized ratio (INR) may take 1 to 8 hours. Interim use of whole blood or component therapy may also be necessary if bleeding is severe.
Phytonadione tablets will not counteract the anticoagulant action of heparin.
When phytonadione tablets are used to correct excessive anticoagulant-induced hypoprothrombinemia, anticoagulant therapy still being indicated, the patient is again faced with the clotting hazards existing prior to starting the anticoagulant therapy. Phytonadione tablets are not a clotting agent, but overzealous therapy with vitamin K1 may restore conditions which originally permitted thromboembolic phenomena. Dosage should be kept as low as possible, and prothrombin time should be checked regularly as clinical conditions indicate.
2.2 Recommended Dosage
Anticoagulant-Induced Prothrombin Deficiency in Adults The recommended dose to correct excessively prolonged prothrombin times caused by oral anticoagulant therapy is, 2.5 mg to 10 mg or up to 25 mg initially. In some instances 50 mg may be required. Frequency and amount of subsequent doses should be determined by prothrombin time response or clinical condition. If, in 12 to 48 hours after oral administration, the prothrombin time has not been shortened satisfactorily, repeat the dose.
Repeated large doses of phytonadione tablets are not warranted in liver disease if the response to initial use of the vitamin is unsatisfactory. Failure to respond to phytonadione may indicate a congenital coagulation defect or that the condition being treated is unresponsive to vitamin K.
Hypoprothrombinemia Due to Other Causes in Adults If possible, discontinuation or reduction of the dosage of drugs interfering with coagulation mechanisms (such as salicylates, antibiotics) is suggested as an alternative to administering concurrent phytonadione tablets. The severity of the coagulation disorder should determine whether the immediate administration of phytonadione tablets are required in addition to discontinuation or reduction of interfering drugs.
The recommended dose is 2.5 mg to 25 mg or more (sometimes up to 50 mg). Evaluate INR after 6 to 8 hours, and repeat dose if INR remains prolonged. Modify subsequent dosage (amount and frequency) based upon the INR or clinical condition.
3 Dosage Forms And Strengths
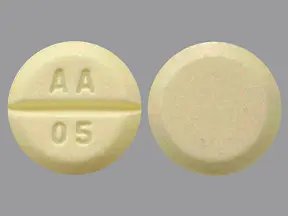
Phytonadione tablets USP, 5 mg are light yellow to yellow colored, round, scored tablets, debossed with ‚ÄúAA‚ÄĚ and ‚Äú05‚ÄĚ on either side of scoring and plain on the other side.
Tablets: 5 mg(3)
4 Contraindications
Phytonadione tablets are contraindicated in patients with a history of a hypersensitivity reaction to phytonadione or inactive ingredients [see Description (11)] .
Hypersensitivity to any component of this medication.(4)
6 Adverse Reactions
The following adverse reactions associated with the use of parenteral phytonadione were identified in clinical studies or post-marketing reports. Because some of these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Severe hypersensitivity reactions, including anaphylactoid reactions and deaths, have been reported following parenteral administration. The majority of these reported events occurred following intravenous administration.
Transient ‚Äúflushing sensations‚ÄĚ and ‚Äúpeculiar‚ÄĚ sensations of taste have been observed with parenteral phytonadione, as well as instances of dizziness, rapid and weak pulse, profuse sweating, brief hypotension, dyspnea, and cyanosis.
Hyperbilirubinemia has been observed in the newborn following administration of parenteral phytonadione. This has occurred primarily with doses above those recommended.
Most common adverse reactions are transient ‚Äúflushing sensations‚ÄĚ, ‚Äúpeculiar‚ÄĚ sensations of taste and instances of dizziness, rapid and weak pulse, profuse sweating, brief hypotension, dyspnea, and cyanosis.(6)
To report SUSPECTED ADVERSE REACTIONS, contact Amneal Pharmaceuticals at 1-877-835-5472 or FDA at 1-800-FDA-1088 orwww.fda.gov/medwatch .
7 Drug Interactions
Anticoagulants Phytonadione may induce temporary resistance to prothrombin-depressing anticoagulants, especially when larger doses of phytonadione are used. Should this occur, higher doses of anticoagulant therapy may be needed when resuming anticoagulant therapy or a change in therapy to a different class of anticoagulant may be necessary (i.e., heparin sodium).
Phytonadione does not affect the anticoagulant action of heparin.
Anticoagulants: May induce temporary resistance to prothrombin-depressing anticoagulants.(7)
8 Use In Specific Populations
8.1 Pregnancy
Risk Summary Published studies with the use of phytonadione during pregnancy have not reported a clear association with phytonadione and adverse developmental outcomes [see Data]. There are maternal and fetal risks associated with vitamin K deficiency during pregnancy [see Clinical Considerations]. Animal reproduction studies have not been conducted with phytonadione.
The estimated background risk for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations Disease-associated maternal and/or embryo/fetal risk
Pregnant women with vitamin K deficiency hypoprothrombinemia may be at increased risk for bleeding diatheses during pregnancy and hemorrhagic events at delivery. Subclinical vitamin K deficiency during pregnancy has been implicated in rare cases of fetal intracranial hemorrhage.
Data Human Data Phytonadione has been measured in cord blood of infants whose mothers were treated with phytonadione during pregnancy in concentrations lower than seen in maternal plasma. Administration of vitamin K1 to pregnant women shortly before delivery increased both maternal and cord blood concentrations. Published data do not report a clear association with phytonadione and adverse maternal or fetal outcomes when used during pregnancy. However, these studies cannot definitively establish the absence of any risk because of methodologic limitations including small sample size and lack of blinding.
Animal Data In pregnant rats receiving vitamin K1 orally, fetal plasma and liver concentrations increased following administration, supporting placental transfer.
8.2 Lactation
Risk Summary Phytonadione is present in breastmilk. There are no data on the effects of phytonadione on the breastfed child or on milk production. The developmental and health benefits of breastfeeding should be considered along with the clinical need for phytonadione and any potential adverse effects on the breastfed child from phytonadione or from the underlying maternal condition.
8.4 Pediatric Use
Safety and effectiveness in pediatric patients have not been established with phytonadione. Hemolysis, jaundice, and hyperbilirubinemia in newborns, particularly in premature infants, have been reported with vitamin K.
8.5 Geriatric Use
Clinical studies of phytonadione did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
11 Description
Phytonadione is a vitamin K replacement, which is a clear, yellow to amber, very viscous, odorless or practically odourless liquid. It is soluble in dehydrated alcohol, benzene, chloroform and ether; slightly soluble in alcohol (96%) and insoluble in water. It has a molecular weight of 450.70 g/mol.
Phytonadione is 1,4-Naphthalenedione, 2-methyl-3-(3,7,11,15-tetramethyl-2-hexadecenyl)-,[R-[R*,R*-(E)]]. Its molecular formula is C 31H 46O 2 and its structural formula is:

Phytonadione tablets, USP for oral administration contain 5 mg of phytonadione, USP and are light yellow to yellow colored, round, scored tablets, debossed with ‚ÄúAA‚ÄĚ and ‚Äú05‚ÄĚ on either side of scoring and plain on the other side. Inactive ingredients are acacia, anhydrous dibasic calcium phosphate, colloidal silicon dioxide, lactose monohydrate, magnesium stearate, pregelatinized starch, and talc.
12 Clinical Pharmacology
12.1 Mechanism of Action
Phytonadione tablets possess the same type and degree of activity as does naturally-occurring vitamin K, which is necessary for the production via the liver of active prothrombin (factor II), proconvertin (factor VII), plasma thromboplastin component (factor IX), and Stuart factor (factor X). The prothrombin test is sensitive to the levels of three of these four factors ‚Äď II, VII, and X. Vitamin K is an essential cofactor for a microsomal enzyme that catalyzes the posttranslational carboxylation of multiple, specific, peptide-bound glutamic acid residues in inactive hepatic precursors of factors II, VII, IX, and X. The resulting gamma-carboxyglutamic acid residues convert the precursors into active coagulation factors that are subsequently secreted by liver cells into the blood.
In normal animals and humans, phytonadione is virtually devoid of pharmacodynamic activity. However, in animals and humans deficient in vitamin K, the pharmacological action of vitamin K is related to its normal physiological function, that is, to promote the hepatic biosynthesis of vitamin K-dependent clotting factors.
12.2 Pharmacodynamics
Phytonadione tablets generally exert their effect within 6 to 10 hours.
12.3 Pharmacokinetics
Absorption Oral phytonadione is adequately absorbed from the gastrointestinal tract only if bile salts are present.
Distribution After absorption, phytonadione is initially concentrated in the liver, but the concentration declines rapidly. Very little vitamin K accumulates in tissues.
Elimination Little is known about the metabolic fate of vitamin K. Almost no free unmetabolized vitamin K appears in bile or urine.
13 Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies of carcinogenicity or impairment of fertility have not been performed with phytonadione. Phytonadione at concentrations up to 2,000 mcg/plate, with or without metabolic activation, was negative in the Ames microbial mutagen test.
16 How Supplied/storage And Handling
Phytonadione Tablets USP, 5 mg are supplied as light yellow to yellow colored, round, scored tablets, debossed with ‚ÄúAA‚ÄĚ and ‚Äú05‚ÄĚ on either side of scoring and plain on the other side.
They are available as follows: Unit dose packages of 20 (2 x 10) NDC 60687-381-94
Storage Store at 20¬į to 25¬įC (68¬į to 77¬įF); excursions permitted between 15¬į to 30¬įC (59¬į to 86¬įF) [see USP Controlled Room Temperature].
Always protect phytonadione tablets, USP from light.
FOR YOUR PROTECTION: Do not use if buler is torn or broken.
17 Patient Counseling Information
Vitamin K1 is fairly rapidly degraded by light; therefore, advise patients to always protect phytonadione tablets from light.
PACKAGING INFORMATION
American Health Packaging unit dose bulers (see How Supplied section) contain drug product from Amneal Pharmaceuticals LLC as follows: (5 mg / 20 UD) NDC 60687-381-94 packaged from NDC 69238-1051
Distributed by: American Health Packaging Columbus, OH 43217
8438194/1022F
Package/label Display Panel Carton 5 Mg

NDC 60687- 381-94
Phytonadione Tablets, USP
5 mg
20 Tablets (2 x 10) ‚ÄÉ‚ÄÉ‚ÄÉ‚ÄÉ‚ÄÉ ‚ÄÉ‚ÄÉ‚ÄÉ‚ÄÉ‚ÄÉ ‚ÄÉ‚ÄÉ Rx Only
Each Tablet Contains: Phytonadione, USP................................................... 5 mg
Usual Dosage: See full prescribing information.
Store at 20¬į to 25¬įC (68¬į to 77¬įF); excursions permitted between 15¬į to 30¬įC (59¬į to 86¬įF) [see USP Controlled Room Temperature].
Protect from light.
FOR YOUR PROTECTION: Do not use if buler is torn or broken.
The drug product contained in this package is from NDC # 69238-1051, Amneal Pharmaceuticals LLC.
Distributed by: American Health Packaging, Columbus, Ohio 43217
738194 0438194/0824
Package/label Display Panel Blister 5 Mg

Phytonadione Tablet, USP
5 mg
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site