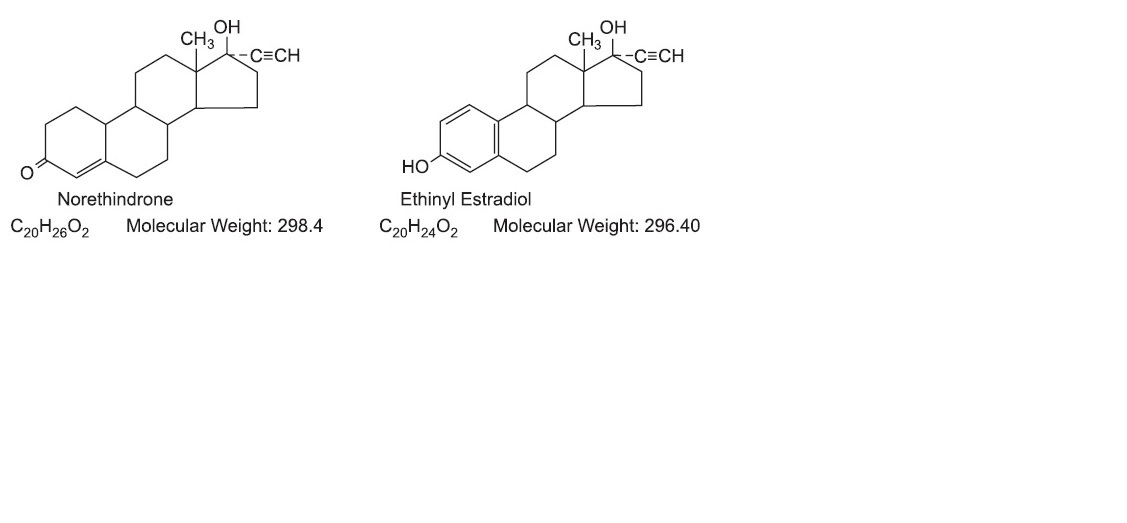
c. Cerebrovascular Diseases
Oral contraceptives have been shown to increase both the relative and attributable risk of cerebrovascular events (thrombotic and hemorrhagic strokes); although, in general, the risk is greatest among older (greater than 35 years), hypertensive women who also smoke. Hypertension was found to be a risk factor for both users and non-users, for both types of strokes, while smoking interacted to increase the risk for hemorrhagic strokes.
In a large study, the relative risk of thrombotic strokes has been shown to range from 3 for normotensive users to 14 for users with severe hypertension. The relative risk of hemorrhagic stroke is reported to be 1.2 for nonsmokers who used oral contraceptives, 2.6 for smokers who did not use oral contraceptives, 7.6 for smokers who used oral contraceptives, 1.8 for normotensive users and 25.7 for users with severe hypertension. The attributable risk is also greater in older women.
d. Dose-Related Risk of Vascular Disease from Oral Contraceptives
A positive association has been observed between the amount of estrogen and progestogen in oral contraceptives and the risk of vascular disease. A decline in serum high density lipoproteins (HDL) has been reported with many progestational agents. A decline in serum high density lipoproteins has been associated with an increased incidence of ischemic heart disease. Because estrogens increase HDL cholesterol, the net effect of an oral contraceptive depends on a balance achieved between doses of estrogen and progestogen and the nature and absolute amount of progestogens used in the contraceptive. The amount of both hormones should be considered in the choice of an oral contraceptive.
Minimizing exposure to estrogen and progestogen is in keeping with good principles of therapeutics. For any particular estrogen/ progestogen combination, the dosage regimen prescribed should be one which contains the least amount of estrogen and progestogen that is compatible with a low failure rate and the needs of the individual patient. New acceptors of oral contraceptive agents should be started on preparations containing 0.05 mg or less of estrogen.
e. Persistence of Risk
There are two studies which have shown persistence of risk of vascular disease for ever-users of oral contraceptives. In a study in the United States, the risk of developing myocardial infarction after discontinuing oral contraceptives persists for at least 9 years for women 40 to 49 years old who had used oral contraceptives for five or more years, but this increased risk was not demonstrated in other age groups. In another study in Great Britain, the risk of developing cerebrovascular disease persisted for at least six years after discontinuation of oral contraceptives, although excess risk was very small. However, both studies were performed with oral contraceptive formulations containing 50 micrograms or higher of estrogens.
2. Estimates of Mortality from Contraceptive Use
One study gathered data from a variety of sources which have estimated the mortality rate associated with different methods of contraception at different ages (Table 2).
TABLE 2 ANNUAL NUMBER OF BIRTH-RELATED OR METHOD-RELATED DEATHS ASSOCIATED WITH CONTROL OF FERTILITY PER 100,000 NONSTERILE WOMEN, BY FERTILITY CONTROL METHOD ACCORDING TO AGE
Ory HW: Mortality associated with fertility and fertility control: 1983. Fam
Plann
Perspect 1983; 15:50-56.
Method 
of 
Control 
and 
Outcome
AGE
15 
to 
19
20 
to 
24
25 
to 
29
30 
to 
34
35 
to 
39
40 
to 
44
No fertility control methodsDeaths are birth related.
7.0
7.4
9.1
14.8
25.7
28.2
Oral contraceptives nonsmokerDeaths are method related.
0.3
0.5
0.9
1.9
13.8
31.6
Oral contraceptives smoker
2.2
3.4
6.6
13.5
51.1
117.2
IUD
0.8
0.8
1.0
1.0
1.4
1.4
Condom
1.1
1.6
0.7
0.2
0.3
0.4
Diaphragm/spermicide
1.9
1.2
1.2
1.3
2.2
2.8
Periodic abstinence
2.5
1.6
1.6
1.7
2.9
3.6
These estimates include the combined risk of death associated with contraceptive methods plus the risk attributable to pregnancy in the event of method failure. Each method of contraception has its specific benefits and risk. The study concluded that with the exception of oral contraceptive users 35 and older who smoke and 40 and older who do not smoke, mortality associated with all methods of birth control is low and below that associated with childbirth.
The observation of a possible increase in risk of mortality with age for oral contraceptive users is based on data gathered in the 1970's‚Äď but not reported until 1983. However, current clinical practice involves the use of lower estrogen dose formulations combined with careful restriction of oral contraceptive use to women who do not have the various risk factors uled in this labeling.
Because of these changes in practice and, also, because of some limited new data which suggest that the risk of cardiovascular disease with the use of oral contraceptives may now be less than previously observed (Porter JB, Hunter J, Jick H, et al. Oral contraceptives and nonfatal vascular disease. Obstet Gynecol 1985;66:1-4 and Porter JB, Jick H, Walker AM. Mortality among oral contraceptive users. Obstet Gynecol 1987;70:29-32), the Fertility and Maternal Health Drugs Advisory Committee was asked to review the topic in 1989. The Committee concluded that although cardiovascular disease risk may be increased with oral contraceptive use after age 40 in healthy nonsmoking women (even with the newer low-dose formulations), there are greater potential health risks associated with pregnancy in older women and with the alternative surgical and medical procedures which may be necessary if such women do not have access to effective and acceptable means of contraception.
Therefore, the Committee recommended that the benefits of oral contraceptive use by healthy nonsmoking women over 40 may outweigh the possible risks. Of course, older women, as all women who take oral contraceptives, should take the lowest possible dose formulation that is effective.
3. Malignant Neoplasms
Breast Cancer
Vyfemla is contraindicated in females who currently have or havehad breast cancer because breast cancer may be hormonally sensitive [see
CONTRAINDICATIONS
].
Epidemiology studies have not found a consistent association between use of combined oral contraceptives (COCs) and breast cancer risk. Studies do not show an association between ever (current or past) use of COCs and risk of breast cancer. However, some studies report a small increase in the risk of breast cancer among current or recent users (<6 months since last use) and current users with longer duration of COC use [see
POSTMARKETING EXPERIENCE].
Cervical cancer 
Some studies suggest that oral contraceptive use has been associated with an increase in the risk of cervical intraepithelial neoplasia in some populations of women.
However, there continues to be controversy about the extent to which such findings may be due to differences in sexual behavior and other factors.
In spite of many studies of the relationship between oral contraceptive use and breast cancer and cervical cancers, a cause-and-effect relationship has not been established.
4. Hepatic Neoplasia
Benign hepatic adenomas are associated with oral contraceptive use, although their occurrence is rare in the United States. Indirect calculations have estimated the attributable risk to be in the range of 3.3 cases/100,000 for users, a risk that increases after four or more years of use. Rupture of hepatic adenomas may cause death through intra-abdominal hemorrhage.
Studies from Britain have shown an increased risk of developing hepatocellular carcinoma in long-term (greater than 8 years) oral contraceptive users. However, these cancers are extremely rare in the U.S. and the attributable risk (the excess incidence) of liver cancers in oral contraceptive users approaches less than one per million users.
5. Risk Of Liver Enzyme Elevations With Concomitant Hepatitis C Treatment
During clinical trials with the Hepatitis C combination drug regimen that contains ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, ALT elevations greater than 5 times the upper limit of normal (ULN), including some cases greater than 20 times the ULN, were significantly more frequent in women using ethinyl estradiol-containing medications such as COCs. Discontinue levonorgestrel and ethinyl estradiol tablets prior to starting therapy with the combination drug regimen ombitasvir/paritaprevir/ritonavir, with or without dasabuvir [see
CONTRAINDICATIONS
]. Levonorgestrel and ethinyl estradiol tablets can be restarted approximately 2 weeks following completion of treatment with the combination drug regimen.
6. Ocular Lesions
There have been clinical case reports of retinal thrombosis associated with the use of oral contraceptives. Oral contraceptives should be discontinued if there is unexplained partial or complete loss of vision; onset of proptosis or diplopia; papilledema; or retinal vascular lesions. Appropriate diagnostic and therapeutic measures should be undertaken immediately.
7. Oral Contraceptive Use Before or During Early Pregnancy
Extensive epidemiological studies have revealed no increased risk of birth defects in women who have used oral contraceptives prior to pregnancy. Studies also do not suggest a teratogenic effect, particularly in so far as cardiac anomalies and limb reduction defects are concerned, when taken inadvertently during early pregnancy.
The administration of oral contraceptives to induce withdrawal bleeding should not be used as a test for pregnancy. Oral contraceptives should not be used during pregnancy to treat threatened or habitual abortion.
It is recommended that for any patient who has missed two consecutive periods, pregnancy should be ruled out before continuing oral contraceptive use. If the patient has not adhered to the prescribed schedule, the possibility of pregnancy should be considered at the time of the first missed period. Oral contraceptive use should be discontinued if pregnancy is confirmed.
8. Gallbladder Disease
Earlier studies have reported an increased lifetime relative risk of gallbladder surgery in users of oral contraceptives and estrogens. More recent studies, however, have shown that the relative risk of developing gallbladder disease among oral contraceptive users may be minimal.
The recent findings of minimal risk may be related to the use of oral contraceptive formulations containing lower hormonal doses of estrogens and progestogens.
9. Carbohydrate and Lipid Metabolic Effects
Oral contraceptives have been shown to cause glucose intolerance in a significant percentage of users. Oral contraceptives containing greater than 75 micrograms of estrogens cause hyperinsulinism, while lower doses of estrogen cause less glucose intolerance. Progestogens increase insulin secretion and create insulin resistance, this effect varying with different progestational agents.
However, in the nondiabetic woman, oral contraceptives appear to have no effect on fasting blood glucose. Because of these demonstrated effects, prediabetic and diabetic women should be carefully observed while taking oral contraceptives.
A small proportion of women will have persistent hypertriglyceridemia while on the pill. As discussed earlier [see
WARNINGS 1.a. 1.d.
], changes in serum triglycerides and lipoprotein levels have been reported in oral contraceptive users.
10. Elevated Blood Pressure
An increase in blood pressure has been reported in women taking oral contraceptives and this increase is more likely in older oral contraceptive users and with continued use. Data from the Royal College of General Practitioners and subsequent randomized trials have shown that the incidence of hypertension increases with increasing concentrations of progestogens.
Women with a history of hypertension or hypertension-related diseases, or renal disease should be encouraged to use another method of contraception. If women elect to use oral contraceptives, they should be monitored closely and if significant elevation of blood pressure occurs, oral contraceptives should be discontinued. For most women, elevated blood pressure will return to normal after stopping oral contraceptives, and there is no difference in the occurrence of hypertension among ever- and never-users.
11. Headache
The onset or exacerbation of migraine or development of headache with a new pattern which is recurrent, persistent or severe requires discontinuation of oral contraceptives and evaluation of the cause.
12. Bleeding Irregularities
Breakthrough bleeding and spotting are sometimes encountered in patients on oral contraceptives, especially during the first three months of use. Nonhormonal causes should be considered and adequate diagnostic measures taken to rule out malignancy or pregnancy in the event of breakthrough bleeding, as in the case of any abnormal vaginal bleeding. If pathology has been excluded, time or a change to another formulation may solve the problem. In the event of amenorrhea, pregnancy should be ruled out.
Women with a history of oligomenorrhea or secondary amenorrhea or young women without regular cycles prior to taking oral contraceptives may again have irregular bleeding or amenorrhea after discontinuation of oral contraceptives.
This product (like all oral contraceptives) is intended to prevent pregnancy. It does not protect against HIV infection (AIDS) and other sexually transmitted diseases.
Oral contraceptives, also known as "birth control pills" or "the pill," are taken to prevent pregnancy and when taken correctly, have a failure rate of about 1% per year when used without missing any pills. The typical failure rate of large numbers of pill users is less than 3% per year when women who miss pills are included.
Oral contraceptive use is associated with certain serious diseases that can be life-threatening or may cause temporary or permanent disability. The risks associated with taking oral contraceptives increase significantly if you:
Smoke
Have high blood pressure, diabetes, high cholesterol
Have or have had clotting disorders, heart attack, stroke, angina pectoris, cancer of the breast or sex organs, jaundice or malignant or benign liver tumors.
You should not take the pill if you suspect you are pregnant or have unexplained vaginal bleeding.
How To Take The Pill
The instructions given in the COMBINATION DETAILED PATIENT LABELING AND BRIEF SUMMARY insert are included inside each foil pouch. The instructions include the directions on starting the first pack on Day-One (first choice) of her period and the Sunday start (Sunday after period starts). The patient is advised that, if she used the Sunday start, she should use a back-up method in the first cycle if she has intercourse before she has taken seven pills. The patient is also instructed as to what she should do if she misses a pill or pills. The patient is warned that she may become pregnant if she misses a pill or pills and that she should use a back-up method of birth control in the event she has intercourse any time during the seven day period following the missed pill or pills.
Instructions on how to use the buler for the (28 Tablets) are included in the
BRIEF SUMMARY PATIENT PACKAGE INSERT
.
This product (like all oral contraceptives) is intended to prevent pregnancy. It does not protect against HIV infection (AIDS) and other sexually transmitted diseases.
Any woman who considers using oral contraceptives (the "birth control pill" or "the pill") should understand the benefits and risks of using this form of birth control.
Although the oral contraceptives have important advantages over other methods of contraception, they have certain risks that no other method has and some of these risks may continue after you have stopped using the oral contraceptive. This brochure will give you much of the information you will need to make this decision and will also help you determine if you are at risk of developing any of the serious side effects of the pill. It will tell you how to use the pill properly so that it will be as effective as possible. However, this brochure is not a replacement for a careful discussion between you and your health care professional.
You should discuss the information provided in this brochure with him or her, both when you first start taking the pill and during your revisits. You should also follow your health care professional's advice with regard to regular check-ups while you are on the pill.
EFFECTIVENESS OF ORAL CONTRACEPTIVES
Oral contraceptives or "birth control pills" or "the pill" are used to prevent pregnancy and are more effective than other nonsurgical methods of birth control. The chance of becoming pregnant is less than 1% (1 pregnancy per 100 women per year of use) when the pills are used correctly and no pills are missed. Typical failure rates are actually 3% per year. The chance of becoming pregnant increases with each missed pill during a menstrual cycle.
In comparison, typical accidental pregnancy rates for other nonsurgical methods of birth control during the first year of use are as follows:
       IUD: 3%
       Diaphragm with spermicides: 18%
       Spermicides alone: 21%
       Vaginal Sponge: 18% to 28%
       Condom alone: 12%
       Periodic abstinence: 20%
       Injectable progestogen: 0.3% to 0.4%
       Implants: 0.03% to 0.04%
       No methods: 85%.
WHO SHOULD NOT TAKE ORAL CONTRACEPTIVES
Some women should not use the pill. For example, you should not take the pill if you are pregnant or think you may be pregnant. You should also not use the pill if you have or have ever had any of the following conditions:
Some women should not use the pill. For example, you should not take the pill if you are pregnant or think you may be pregnant. You should also not use the pill if you have or have ever had any of the following conditions:
A history of heart attack or stroke
Blood clots in the legs (thrombophlebitis), lungs (pulmonary embolism), or eyes
A history of blood clots in the deep veins of your legs
Chest pain (angina pectoris)
Known or suspected breast cancer or cancer of the lining of the uterus
Unexplained vaginal bleeding (until a diagnosis is reached by your doctor)
Yellowing of the whites of the eyes or of the skin (jaundice) during pregnancy or during previous use of the pill
Liver tumor (benign or cancerous)
Take any Hepatitis C drug combination containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir. This may increase levels of the liver enzyme "alanine aminotransferase" (ALT) in the blood.
Tell your health care professional if you have ever had any of these conditions. Your health care professional can recommend a safer method of birth control.
OTHER CONSIDERATIONS BEFORE TAKING ORAL CONTRACEPTIVES
Tell your health care professional if you have:
Breast nodules, fibrocystic disease of the breast or an abnormal breast x-ray or mammogram
Diabetes
Elevated cholesterol or triglycerides
High blood pressure
Migraine or other headaches or epilepsy
Mental depression
Gallbladder, heart, or kidney disease
History of scanty or irregular menstrual periods
Women with any of these conditions should be checked often by their health care professional if they choose to use oral contraceptives.
Also, be sure to inform your doctor or health care professional if you smoke or are on any medications.
RISKS OF TAKING ORAL CONTRACEPTIVES
1. Risk of Developing Blood Clots
Blood clots and blockage of blood vessels are the most serious side effects of taking oral contraceptives. In particular, a clot in the legs can cause thrombophlebitis and a clot that travels to the lungs can cause a sudden blocking of the vessel carrying blood to the lungs. Either of these can cause death or disability. Rarely, clots occur in the blood vessels of the eye and may cause blindness, double vision, or impaired vision.
If you take oral contraceptives and need elective surgery, need to stay in bed for a prolonged illness, or have recently delivered a baby, you may be at risk of developing blood clots. You should consult your doctor about stopping oral contraceptives three to four weeks before surgery and not taking oral contraceptives for two weeks after surgery or during bed rest. You should also not take oral contraceptives soon after delivery of a baby. It is advisable to wait for at least four weeks after delivery if you are not breastfeeding. If you are breastfeeding see the section on Breastfeeding in
GENERAL PRECAUTIONS
.
2. Heart Attacks and Strokes
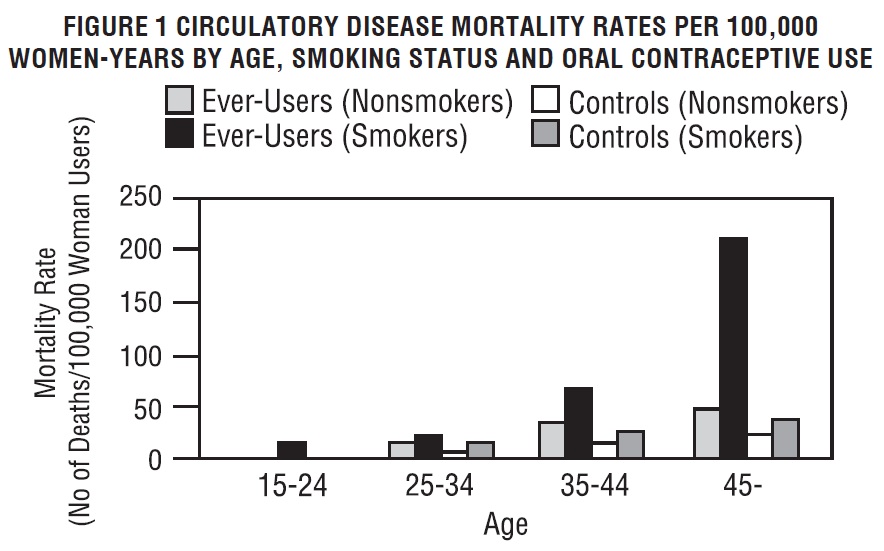
Oral contraceptives may increase the tendency to develop strokes (stoppage or rupture of blood vessels in the brain) and angina pectoris and heart attacks (blockage of blood vessels in the heart). Any of these conditions can cause death or disability. Smoking greatly increases the possibility of suffering heart attacks and strokes. Furthermore, smoking and the use of oral contraceptives greatly increase the chances of developing and dying of heart disease.
3. Gallbladder Disease
Oral contraceptive users probably have a greater risk than nonusers of having gallbladder disease, although this risk may be related to pills containing high doses of estrogens.
4. Liver Tumors
In rare cases, oral contraceptives can cause benign but dangerous liver tumors. These benign liver tumors can rupture and cause fatal internal bleeding. In addition, a possible, but not definite, association has been found with the pill and liver cancers in two studies, in which a few women who developed these very rare cancers were found to have used oral contraceptives for long periods. However, liver cancers in general are extremely rare and the chance of developing liver cancer from using the pill is thus even rarer.
5. Risk of Cancer
It is not known if hormonal birth control pills causes breast cancer. Some studies, but not all, suggest that there could be a slight increase in the risk of breast cancer among current users with longer duration of use.
If you have breast cancer now, or have had it in the past, do not use hormonal birth control because some breast cancers are sensitive to hormones.
Some studies have found an increase in the incidence of cancer of the cervix in women who use oral contraceptives. However, this finding may be related to factors other than the use of oral contraceptives.
ESTIMATED RISK OF DEATH FROM A BIRTH CONTROL METHOD OR PREGNANCY
All methods of birth control and pregnancy are associated with a risk of developing certain diseases which may lead to disability or death. An estimate of the number of deaths associated with different methods of birth control and pregnancy has been calculated and is shown in the following table.
ANNUAL NUMBER OF BIRTH-RELATED OR METHOD-RELATED DEATHS ASSOCIATED WITH CONTROL OF FERTILITY PER 100,000 NONSTERILE WOMEN, BY FERTILITY CONTROL METHOD ACCORDING TO AGE
Ory HW: Mortality associated with fertility and fertility control: 1983. Fam
Plann
Perspect 1983; 15:50-56.
Method 
of 
Control 
AGE
and 
Outcome
15 
to 
19
20 
to 
24
25 
to 
29
30 
to 
34
35 
to 
39
40 
to 
44
No fertility control methodsDeaths are birth related.
7.0
7.4
9.1
14.8
25.7
28.2
Oral contraceptives non-smokerDeaths are method related.
0.3
0.5
0.9
1.9
13.8
31.6
Oral contraceptives smoker
2.2
3.4
6.6
13.5
51.1
117.2
IUD
0.8
0.8
1.0
1.0
1.4
1.4
Condom
1.1
1.6
0.7
0.2
0.3
0.4
Diaphragm/spermicide
1.9
1.2
1.2
1.3
2.2
2.8
Periodic abstinence
2.5
1.6
1.6
1.7
2.9
3.6
It can be seen in the table that for women aged 15 to 39, the risk of death was highest with pregnancy (7-26 deaths per 100,000 women, depending on age). Among pill users who do not smoke, the risk of death was always lower than that associated with pregnancy for any age group, although over the age of 40, the risk increases to 32 deaths per 100,000 women, compared to 28 associated with pregnancy at that age. However, for pill users who smoke and are over the age of 35, the estimated number of deaths exceeds those for other methods of birth control. If a woman is over the age of 40 and smokes, her estimated risk of death is four times higher (117/100,000 women) than the estimated risk associated with pregnancy (28/100,000 women) in that age group.
The suggestion that women over 40 who don't smoke should not take oral contraceptives is based on information from older high-dose pills and on less selective use of pills than is practiced today. An Advisory Committee of the FDA discussed this issue in 1989 and recommended that the benefits of oral contraceptive use by healthy, nonsmoking women over 40 years of age may outweigh the possible risks. However, all women, especially older women, are cautioned to use the lowest dose pill that is effective.
In the above table, the risk of death from any birth control method is less than the risk of childbirth, except for oral contraceptive users over the age of 35 who smoke and pill users over the age of 40 even if they do not smoke.
You should discuss this information with your health care professional.
WARNING SIGNALS
If any of these adverse conditions occur while you are taking oral contraceptives, call your doctor immediately:
Sharp chest pain, coughing of blood, or sudden shortness of breath (indicating a possible clot in the lung)
Pain in the calf (indicating a possible clot in the leg)
Crushing chest pain or heaviness in the chest (indicating a possible heart attack)
Sudden severe headache or vomiting, dizziness or fainting, disturbances of vision or speech, weakness, or numbness in an arm or leg (indicating a possible stroke)
Sudden partial or complete loss of vision (indicating a possible clot in the eye)
Breast lumps (indicating possible breast cancer or fibrocystic disease of the breast; ask your doctor or healthcare provider to show you how to examine your breasts)
Severe pain or tenderness in the stomach area (indicating a possibly ruptured liver tumor)
Difficulty in sleeping, weakness, lack of energy, fatigue, or change in mood (possibly indicating severe depression)
Jaundice or a yellowing of the skin or eyeballs, accompanied frequently by fever, fatigue, loss of appetite, dark-colored urine, or light-colored bowel movements (indicating possible liver problems)
Abnormal vaginal bleeding [see
SIDE EFFECTS OF ORAL CONTRACEPTIVES, 1. Vaginal Bleeding
below.]
SIDE EFFECTS OF ORAL CONTRACEPTIVES
In addition to the risks and more serious side effects discussed above [see
RISKS OF TAKING ORAL CONTRACEPTIVES
,
ESTIMATED RISK OF DEATH FROM A BIRTH CONTROL METHOD OR PREGNANCY
and
WARNING SIGNALS
sections), the following may also occur:
1. Vaginal Bleeding
Irregular vaginal bleeding or spotting may occur while you are taking the pills. Irregular bleeding may vary from slight staining between menstrual periods to breakthrough bleeding which is a flow much like a regular period. Irregular bleeding occurs most often during the first few months of oral contraceptive use, but may also occur after you have been taking the pill for some time.
Such bleeding may be temporary and usually does not indicate any serious problems. It is important to continue taking your pills on schedule. If the bleeding occurs in more than one cycle or lasts for more than a few days, talk to your doctor or health care provider.
2. Gastrointestinal Effects
The most frequent, unpleasant side effects are nausea and vomiting, stomach cramps, bloating, and a change in appetite.
3. Contact Lenses
If you wear contact lenses and notice a change in vision or an inability to wear your lenses, contact your doctor or health care provider.
4. Fluid Retention
Oral contraceptives may cause edema (fluid retention) with swelling of the fingers or ankles and may raise your blood pressure. If you experience fluid retention, contact your doctor or health care professional.
5. Melasma
A spotty darkening of the skin is possible, particularly of the face.
6. Other Side Effects
Other side effects may include change in appetite, headache, nervousness, depression, dizziness, loss of scalp hair, rash, and vaginal infections.
If any of these side effects bother you, call your doctor or health care professional.
1. Missed Periods and Use of Oral Contraceptives Before or During Early Pregnancy
There may be times when you may not menstruate regularly after you have completed taking a cycle of pills. If you have taken your pills regularly and miss one menstrual period, continue taking your pills for the next cycle but be sure to inform your health care professional before doing so. If you have not taken the pills daily as instructed and missed a menstrual period, or if you missed two consecutive menstrual periods, you may be pregnant. Check with your health care professional immediately to determine whether you are pregnant. Do not continue to take oral contraceptives until you are sure you are not pregnant, but continue to use another method of contraception.
There is no conclusive evidence that oral contraceptive use is associated with an increase in birth defects, when taken inadvertently during early pregnancy. Previously, a few studies had reported that oral contraceptives might be associated with birth defects, but these studies have not been confirmed. Nevertheless, oral contraceptives or any other drugs should not be used during pregnancy unless clearly necessary and prescribed by your doctor. You should check with your doctor about risks to your unborn child of any medication taken during pregnancy.
2. While Breastfeeding
If you are breastfeeding, consult your doctor before starting oral contraceptives. Some of the drug will be passed on to the child in the milk. A few adverse effects on the child have been reported, including yellowing of the skin (jaundice) and breast enlargement. In addition, oral contraceptives may decrease the amount and quality of your milk. If possible, do not use oral contraceptives while breastfeeding. You should use another method of contraception since breastfeeding provides only partial protection from becoming pregnant and this partial protection decreases significantly as you breastfeed for longer periods of time. You should consider starting oral contraceptives only after you have weaned your child completely.
3. Laboratory Tests
If you are scheduled for any laboratory tests, tell your doctor you are taking birth control pills. Certain blood tests may be affected by birth control pills.
4. Drug Interactions
Certain drugs may interact with birth control pills to make them less effective in preventing pregnancy or cause an increase in breakthrough bleeding. Such drugs include rifampin, drugs used for epilepsy such as barbiturates (for example, phenobarbital) and phenytoin (Dilantin is one brand of this drug), phenylbutazone (Butazolidin is one brand) and possibly ampicillin and tetracyclines (several brand names). You may need to use an additional method of contraception when you take drugs which can make oral contraceptives less effective.
HOW TO TAKE THE PILL
IMPORTANT 
POINTS 
TO 
REMEMBER
SEXUALLY-TRANSMITTED DISEASES
This product (like all oral contraceptives) is intended to prevent pregnancy. It does not protect against transmission of HIV (AIDS) and other sexually transmitted diseases such as Chlamydia, genital herpes, genital warts, gonorrhea, hepatitis B, and syphilis.
BEFORE
YOU START TAKING YOUR PILLS:
BE SURE TO READ THESE DIRECTIONS:
Before you start taking your pills.
Anytime you are not sure what to do.
THE RIGHT WAY TO TAKE THE PILL IS TO TAKE ONE PILL EVERY DAY AT THE SAME TIME.
If you miss pills you could get pregnant. This includes starting the pack late. The more pills you miss, the more likely you are to get pregnant.
MANY WOMEN HAVE SPOTTING OR LIGHT BLEEDING, OR MAY FEEL SICK TO THEIR STOMACH DURING THE FIRST 1 to 3 PACKS OF PILLS.
If you do feel sick to your stomach, do not stop taking the pill. The problem will usually go away. If it doesn't go away, check with your doctor or clinic.
MISSING PILLS CAN ALSO CAUSE SPOTTING OR LIGHT BLEEDING, even when you make up these missed pills.
On the days you take 2 pills to make up for missed pills, you could also feel a little sick to your stomach.
IF YOU HAVE VOMITING OR DIARRHEA, for any reasons, or IF YOU TAKE SOME MEDICINES, including some antibiotics, your pills may not work as well.
Use a back-up method (such as condoms, foam, or sponge) until you check with your doctor or clinic.
IF YOU HAVE TROUBLE REMEMBERING TO TAKE THE PILL, talk to your doctor or clinic about how to make pill-taking easier or about using another method of birth control.
IF YOU HAVE ANY QUESTIONS OR ARE UNSURE ABOUT THE INFORMATION IN THIS LEAFLET, call your doctor or clinic.
BEFORE 
YOU 
START 
TAKING 
YOUR 
PILLS
DECIDE WHAT TIME OF DAY YOU WANT TO TAKE YOUR PILL.
It is important to take it at about the same time every day.
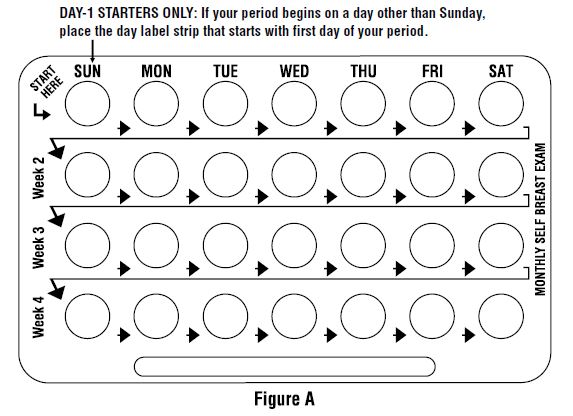
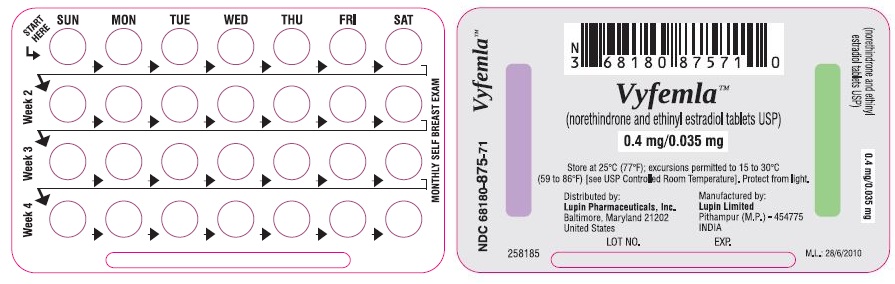
LOOK AT YOUR PILL PACK TO SEE IF IT HAS 28 PILLS:
The 28-pill pack has 21 "active" light peach pills (with hormones) to take for 3 weeks, followed by 1 week of reminder white pills (without hormones).
Refer to the sample of the buler below.
For use of day labels, see WHEN TO START THE
FIRST
PACK OF PILLS
3. BE SURE YOU HAVE READY AT ALL TIMES:
ANOTHER KIND OF BIRTH CONTROL (such as condoms, foam, or sponge) to use as a back-up in case you miss pills.
An EXTRA, FULL PILL PACK.
WHEN 
TO 
START 
THE 
FIRST 
PACK 
OF 
PILLS
You have a choice of which day to start taking your first pack of pills. Vyfemla‚ĄĘ (norethindrone and ethinyl estradiol tablets USP) is available in Buler which is designed for Sunday Start. Day 1 Start is also provided. Decide with your doctor or clinic which is the best day for you. Pick a time of day which will be easy to remember.
DAY-1 START:
Take the first "active" light peach pill of the first pack during the first 24 hours of your period.
You will not need to use a back-up method of birth control, since you are starting the pill at the beginning of your period.
SUNDAY START:
Take the first "active" light peach pill of the first pack on the Sunday after your period starts, even if you are still bleeding. If your period begins on Sunday, start the pack that same day.
Use another method of birth control as a back-up method if you have sex anytime from the Sunday you start your first pack until the next Sunday (7 days). Condoms, foam, or the sponge are good back-up methods of birth control.
WHAT 
TO 
DO 
DURING 
THE 
MONTH
1. TAKE ONE PILL AT THE SAME TIME EVERY DAY UNTIL THE PACK IS EMPTY.
Do not skip pills even if you are spotting or bleeding between monthly periods or feel sick to your stomach (nausea).
Do not skip pills even if you do not have sex very often.
2. WHEN YOU FINISH A PACK OR SWITCH YOUR BRAND OF PILLS:
28 pills: Start the next pack on the day after your last "reminder" pill. Do not wait any days between packs.
WHAT 
TO 
DO 
IF 
YOU 
MISS 
PILLS
If you MISS 1 light peach "active" pill:
Take it as soon as you remember. Take the next pill at your regular time. This means you may take 2 pills in 1 day.
You do not need to use a back-up birth control method if you have sex.
If you MISS 2 light peach "active" pills in a row in WEEK 1 OR WEEK 2 of your pack:
Take 2 pills on the day you remember and 2 pills the next day.
Then take 1 pill a day until you finish the pack.
You MAY BECOME PREGNANT if you have sex in the 7 days after you miss pills. You MUST use another birth control method (such as condoms, foam, or sponge) as a back-up for those 7 days.
If you MISS 2 light peach "active" pills in a row in THE 3rd WEEK:
1.       If you are a Day 1 Starter:
                   THROW OUT the rest of the pill pack and start a new pack that same day.
                   If you are a Sunday Starter:
                   Keep taking 1 pill every day until Sunday.
                   On Sunday, THROW OUT the rest of the pack and start a new pack of pills that same day.
2.       You may not have your period this month but this is expected. However, if you miss your period 2 months in a row, call your doctor or clinic because you might be pregnant.
3.       You MAY BECOME PREGNANT if you have sex in the 7 days after you miss pills. You MUST use another birth control method (such as condoms, foam, or sponge) as a back-up for those 7 days.
If you MISS 3 OR MORE light peach "active" pills in a row (during the first 3 weeks):
1.       If you are a Day 1 Starter:
                   THROW OUT the rest of the pill pack and start a new pack that same day.
                   If you are a Sunday Starter:
                   Keep taking 1 pill every day until Sunday.
                   On Sunday, THROW OUT the rest of the pack and start a new pack of pills that same day.
2.       You may not have your period this month but this is expected. However, if you miss your period 2 months in a row, call your doctor or clinic because you might be pregnant.
3.       You MAY BECOME PREGNANT if you have sex in the 7 days after you miss pills. You MUST use another birth control method (such as condoms, foam, or sponge) as a back-up for those 7 days.
A 
REMINDER 
FOR 
THOSE 
ON 
28
-
DAY 
PACKS
:
If you forget any of the 7 white "reminder" pills in Week 4:THROW AWAY the pills you missed.Keep taking 1 pill each day until the pack is empty. You do not need a back-up method.
FINALLY
, 
IF 
YOU 
ARE 
STILL 
NOT 
SURE 
WHAT 
TO 
DO 
ABOUT 
THE 
PILLS 
YOU 
HAVE 
MISSED
: 
Use¬†a¬†BACK-UP¬†METHOD¬†anytime¬†you¬†have¬†sex.KEEP¬†TAKING¬†ONE¬†‚ÄúACTIVE‚Ä̬†PILL¬†EACH¬†DAY¬†until¬†you¬†can¬†reach¬†your¬†doctor¬†or¬†clinic.