Zeniquin (marbofloxacin 200 mg) Dailymed
Generic: marbofloxacin
IMPRINT: PFIZER 25 MG ; 25 MG LOGO
SHAPE: oval
COLOR: brown SCORE: 2
All Imprints
marbofloxacin 25 mg - pfizer 25 mg 25 mg logo oval brown
zeniquin (marbofloxacin) tablet - pfizer 25 mg oval brown
Go PRO for all pill images
Tablets
For oral use in dogs and cats only
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Federal law prohibits the extralabel use of this drug in food-producing animals.
Description
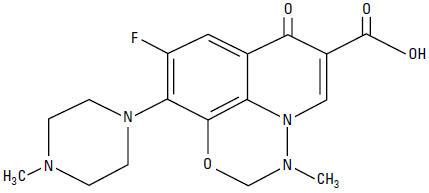
Marbofloxacin is a synthetic broad-spectrum antibacterial agent from the fluoroquinolone class of chemotherapeutic agents. Marbofloxacin is the non-proprietary designation for 9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido[3,2,1-ij][4,1,2] benzoxadiazine-6-carboxylic acid. The empirical formula is C17H19FN4O4 and the molecular weight is 362.36. The compound is soluble in water; however, solubility decreases in alkaline conditions. The N-octanol/water partition coefficient (Kow) is 0.835 measured at pH 7 and 25°C.
Figure 1: Chemical structure of marbofloxacin
Clinical Pharmacology
Marbofloxacin is rapidly and almost completely absorbed from the gastrointestinal tract following oral administration to fasted animals. Divalent cations are generally known to diminish the absorption of fluoroquinolones. The effects of concomitant feeding on the absorption of marbofloxacin have not been determined. (See Drug Interactions.) In the dog, approximately 40% of an oral dose of marbofloxacin is excreted unchanged in the urine1. Excretion in the feces, also as unchanged drug, is the other major route of elimination in dogs. Ten to 15% of marbofloxacin is metabolized by the liver in dogs.
In vitro plasma protein binding of marbofloxacin in dogs was 9.1% and in cats was 7.3%. In the cat, approximately 70% of an oral dose is excreted in the urine as marbofloxacin and metabolites with approximately 85% of the excreted material as unchanged drug. Pharmacokinetic parameters related to intravenous dosing were estimated in a study of 6 healthy adult beagle dogs, and are summarized in Table 1. The absolute bioavailability following dosing of oral tablets to the same animals was 94%.
Marbofloxacin plasma concentrations were determined over time in healthy adult beagle dogs (6 dogs per dosage group) following single oral doses of 1.25 mg/lb or 2.5 mg/lb. Absorption of orally administered marbofloxacin increases proportionally over the dose range of 1.25 to 2.5 mg/lb. Marbofloxacin plasma concentrations were determined over time in 7 healthy adult male cats following a single oral dose of 2.5 mg/lb. Plasma pharmacokinetic parameters following oral dosing of dogs and cats are summarized in Figures 2 and 3 and in Table 2. Based on the terminal elimination half-life and the dosing interval, steady-state levels are reached after the third dose and are expected to be approximately 25% greater in dogs and 35% greater in cats than those achieved after a single dose. Marbofloxacin is widely distributed in canine tissues. Tissue concentrations of marbofloxacin were determined in healthy male beagle dogs (4 dogs per time period) at 2, 18 and 24 hours after a single oral dose (1.25 or 2.5 mg/lb) and are summarized in Tables 3a and 3b.
Table 1:Â Mean pharmacokinetic parameters following intravenous administration of marbofloxacin to 6 adult beagle dogs at a dosage of 2.5 mg/lb.
Parameter
Estimate ± SD* n=6
 Total body clearance, (mL/h•kg)  94 ± 8  Volume of distribution at steady state, Vss, (L/kg) 1.19 ± 0.08  AUC0-inf (μg•h/mL) 59 ± 5  Terminal plasma elimination half-life, t1/2(h) 9.5 ± 0.7
* SD = standard deviation
Table 2: Mean pharmacokinetic parameters following oral administration of marbofloxacin tablets to adult beagle dogs at a nominal dosage of 1.25 mg/lb or2.5 mg/lb and to cats at 2.5 mg/lb.â€
Parameters Dog Estimate ± SD* (1.25 mg/lb) n=6 Dog Estimate ± SD* (2.5 mg/lb) n=6 Cat Estimate ± SD* (2.5 mg/lb) n=7 Time of maximum concentration, Tmax(h)  1.5 ± 0.3  1.8 ± 0.3  1.2 ± 0.6  Maximum concentration, Cmax, (μg/mL)  2.0 ± 0.2  4.2 ± 0.5  4.8 ± 0.7  AUC0-inf (μg•h/mL)  31.2 ± 1.6  64 ± 8  70 ± 6  Terminal plasma elimination half-life, t1/2(h)  10.7 ± 1.6  10.9 ± 0.6  12.7 ± 1.1
†mean actual dosages administered to dogs were 1.22 mg/lb and 2.56 mg/lb, respectively, and the meanactual dosage administered to cats was 2.82 mg/lb.* SD = standard deviation
Figure 2: Mean plasma concentrations (µg/mL) following single oral administration of marbofloxacin to adult beagle dogs at dosages of 1.25 mg/lb or 2.5 mg/lb.
* See Table 4 in Microbiology section for MIC data.

Figure 3: Mean plasma concentrations (µg/mL) following single oral administration of marbofloxacin to adult cats at a dosage of 2.5 mg/lb.
* See Table 5 in Microbiology section for MIC data.
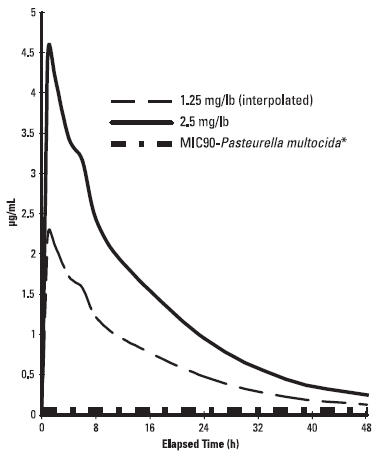
Table 3a: Tissue distribution following a single oral administration of marbofloxacin tablets to adult beagle dogs at a dosage of 1.25 mg/lb*.
Tissue Marbofloxacin 2 hours (n=4) Concentrations 18 hours (n=4) (μg/g ± SD) 24 hours (n=4) bladder  4.8 ± 1.1  2.6 ± 1.5  1.11 ± 0.19 bone marrow  3.1 ± 0.5  1.5 ± 1.5  0.7 ± 0.2 feces  15 ± 9  48 ± 40  26 ± 11 jejunum  3.6 ± 0.5  1.3 ± 1.0  0.7 ± 0.3 kidney  7.1 ±1.7  1.4 ± 0.5  0.9 ± 0.3 lung  3.0 ± 0.5  0.8 ± 0.2  0.57 ± 0.19 lymph node  5.5 ± 1.1  1.3 ± 0.3  1.0 ± 0.3 muscle  4.1 ± 0.3  1.0 ± 0.3  0.7 ± 0.2 prostate  5.6 ± 1.4  1.8 ± 0.6  1.1 ± 0.4 skin  1.9 ± 0.6  0.41 ± 0.13  0.32 ± 0.08
* SD = standard deviation
Table 3b: Tissue distribution following a single oral administration of marbofloxacin tablets to adult beagle dogs at a dosage of 2.5 mg/lb.
Tissue Marbofloxacin 2 hours (n=4) Concentrations 18 hours (n=4) (μg/g ± SD*) 24 hours (n=4) bladder 12 ± 4 6 ± 7 1.8 ± 0.4 bone marrow 4.6 ± 1.5 1.28 ± 0.13 0.9 ± 0.3 feces 18 ± 3 52 ± 17 47 ± 28 jejunum 7.8 ± 1.1 2.0 ± 0.3 1.1 ± 0.3 kidney 12.7 ±1.7 2.7 ± 0.3 1.6 ± 0.2 lung 5.48 ± 0.17 1.45 ± 0.19 1.0 ± 0.2 lymph node 8.3 ± 0.7 2.3 ± 0.5 2.03 ± 0.06 muscle 7.5 ± 0.5 1.8 ± 0.3 1.20 ± 0.12 prostate 11 ± 3 2.7 ± 1.0 2.0 ± 0.5 skin 3.20 ± 0.33 0.705 ± 0.013 0.46 ± 0.09
* SD = standard deviation
Microbiology
The primary action of fluoroquinolones is to inhibit the bacterial enzyme, DNA gyrase. In susceptible organisms, fluoroquinolones are rapidly bactericidal at relatively low concentrations. Marbofloxacin is bactericidal against a broad range of gram-negative and gram-positive organisms. The minimum inhibitory concentrations (MICs) of pathogens isolated in clinical field studies performed in the United States were determined using National Committee for Clinical Laboratory Standards (NCCLS) standards, and are shown in Tables 4 and 5.
Table 4. MIC Values* (μg/mL) of marbofloxacin against pathogens isolated from skin, soft tissue and urinary tract infections in dogs enrolled in clinical studies conducted during 1994–1996.
Organism No. of Isolates MIC50 MIC90 MIC Range Staphylococcus intermedius 135 0.25 0.25 0.125-2
Escherichia coli
61 0.03 0.06 0.015-2 Proteus mirabilis 35 0.06 0.125 0.03-0.25
Beta-hemolytic Streptococcus,
(not Group A or Group B)
25 1 2 0.5-16
Streptococcus,
Group D enterococcus
16 1 4 0.008-4
Pasteurella multocida
13 0.015 0.06 ≤0.008-0.5 Staphylococcus aureus 12 0.25 0.25 0.25-0.5 Enterococcus faecalis 11 2 2 1-4 Klebsiella pneumonia 11 0.06 0.06 0.01-0.06 Pseudomonas spp. 9 ** ** 0.06-1 Pseudomonas aeruginosa  7 ** ** 0.25-1
* The correlation between in vitro susceptibility data (MIC) and clinical response has not beendetermined.** MIC50 and MIC90 not calculated due to insufficient number of isolates.
Table 5: MIC Values* (μg/mL) of marbofloxacin against pathogens isolated fromskin and soft tissue infections in cats enrolled in clinical studies conducted in 1995and 1998.
Organism No. of Isolates MIC50 MIC90 MIC Range
Pasteurella multocida
135 0.03 0.06 ≤0.008-0.25 Beta-hemolytic Streptococcus 22 1 1 0.06-1 Staphylococcus aureus 21 0.25 0.5 0.125-1 Corynebacterium spp. 14 0.5 1 0.25-2 Staphylococcus intermedius 11 0.25 0.5 0.03-0.5 Enterococcus faecalis 10 2.0 2.0 1.0-2.0 Escherichia coli 10 0.03 0.03 0.015-0.03
Bacillus spp.
10 0.25 0.25 0.125-0.25
* The correlation between in vitro susceptibility data (MIC) and clinical response has not beendetermined.
Indications And Usage
Zeniquin (marbofloxacin) tablets are indicated for the treatment of infections in dogs and cats associated with bacteria susceptible to marbofloxacin.
Contraindications
Marbofloxacin and other quinolones have been shown to cause arthropathy in immature animals of most species tested, the dog being particularly sensitive to this side effect. Marbofloxacin is contraindicated in immature dogs during the rapid growth phase (small and medium breeds up to 8 months of age, large breeds up to 12 months of age and giant breeds up to 18 months of age). Marbofloxacin is contraindicated in cats under 12 months of age. Marbofloxacin is contraindicated in dogs and cats known to be hypersensitive to quinolones.
Warning
For use in animals only. Keep out of reach of children. Avoid contact with eyes. In case of contact, immediately flush eyes with copious amounts of water for 15 minutes. In case of dermal contact, wash skin with soap and water. Consult a physician if irritation persists following ocular or dermal exposure. Individuals with a history of hypersensitivity to fluoroquinolones should avoid this product. In humans, there is a risk of user photosensitization within a few hours after excessive exposure to quinolones. If excessive accidental exposure occurs, avoid direct sunlight.
Precautions
Quinolones should be used with caution in animals with known or suspected central nervous system (CNS) disorders. In such animals, quinolones have, in rare instances, been associated with CNS stimulation which may lead to convulsive seizures. Quinolones have been shown to produce erosions of cartilage of weight-bearing joints and other signs of arthropathy in immature animals of various species. The use of fluoroquinolones in cats has been reported to adversely affect the retina. Such products should be used with caution in cats. The safety of marbofloxacin in animals used for breeding purposes, pregnant, or lactating has not been demonstrated.
Adverse Reactions
The following clinical signs were reported during the course of clinical field studies in dogs receiving marbofloxacin at dosages up to 2.5 mg/lb daily: decreased or loss of appetite (5.4%), decreased activity (4.4%), and vomiting (2.9%). The following signs were reported in less than 1% of cases in dogs: increased thirst, soft stool/diarrhea, behavioral changes, shivering/shaking/tremors, and ataxia. One dog which had a seizure the day before study enrollment experienced a seizure while on marbofloxacin therapy.
The following clinical signs were reported during clinical field studies in cats receiving 1.25 mg/lb/day: diarrhea (2.1%) and soft stool (1.4%). Vomiting was reported in less than 1% of cases in cats.
Dosage And Administration
The recommended dosage for oral administration to dogs and cats is 1.25 mg marbofloxacin per lb of body weight once daily, but the dosage may be safely increased to 2.5 mg/lb.
For the treatment of skin and soft tissue infections, Zeniquin tablets should be given for 2–3 days beyond the cessation of clinical signs for a maximum of 30 days. For the treatment of urinary tract infections, Zeniquin tablets should be administered for at least 10 days. If no improvement is noted within 5 days, the diagnosis should be re-evaluated and a different course of therapy considered.
Drug Interactions
Compounds (e.g., sucralfate, antacids, and mineral supplements) containing divalent and trivalent cations (e.g., iron, aluminum, calcium, magnesium, and zinc) can interfere with the absorption of quinolones which may result in a decrease in product bioavailability. Therefore, the concomitant oral administration of quinolones with foods, supplements, or other preparations containing these compounds should be avoided.
Effectiveness Confirmation
Clinical effectiveness was confirmed in bacterial skin and soft tissue infections in dogs and cats and urinary tract infections (cystitis) in dogs associated with bacteria susceptible to marbofloxacin. Bacterial pathogens isolated in clinical field studies are provided in the Microbiology section.
Target Animal Safety
Dogs
The toxicity of marbofloxacin was assessed in 12- to 14-month-old beagle dogs administered marbofloxacin at 2.5, 7.5 and 12.5 mg/lb/day for 42 days. Vomiting, reddened skin (usually involving the ears) and reddened mucous membranes were occasionally observed in all groups, including controls, but were noted most frequently in the 12.5 mg/lb group. Decreased food consumption and weight loss were significant in the 7.5 mg/lb and 12.5 mg/lb groups. No clinical lameness was noted in any of the treated animals. Minimal to slight lesions in the articular cartilage were observed in 1/8 placebo-treated animals and in 3/8 animals given 12.5 mg marbofloxacin/lb. Macroscopically, these lesions were vesicles, raised areas, or depressed, light-colored areas. Microscopically, these lesions were characterized by the presence of one or more of the following: fissuring, erosion, chondrocyte proliferation, fibrillation, or vertical splitting of the articular cartilage. These cartilage lesions in treated dogs were similar to those in control dogs, and were not typical of those produced by fluoroquinolones. In addition to the above pathologic alterations, red areas of articular cartilage were noted macroscopically in 0/8 placebo-treated dogs and in 2/8 dogs from each of the three marbofloxacin-treated groups. These areas usually correlated microscopically with areas of vascularity of the articular surface, but could not be confirmed microscopically in all animals. They consisted of large blood vessels in mature fibrous connective tissue, with no indication of active vascularization due to drug-induced damage. They were considered most likely to be developmental anomalies or normal variations of the joint surface and were not considered to be related to drug treatment.
Marbofloxacin was administered to 12- to 14-month-old beagle dogs at a dosage of 25 mg/lb/day for 12 days. Decreased food consumption, vomiting, dehydration, excessive salivation, tremors, reddened skin, facial swelling, decreased activity and weight loss were seen in treated dogs. No clinical lameness was noted. As in the 42 day study, grossly visible, focal, red areas of articular cartilage were seen. These findings were noted in 2/6 placebo-treated dogs and in 4/6 marbofloxacintreated dogs. The foci were areas of fibrocartilage with prominent vascularization or increased vascularization of subchondral bone. Due to the appearance microscopically and macroscopically, these red foci were described as likely to be developmental anomalies or normal variations in articular cartilage.
Marbofloxacin administered to 3- to 4-month-old, large breed, purpose-bred mongrel dogs at a dosage of 5 mg/lb/day for 14 days resulted in marked lameness in all dogs due to articular cartilage lesions. Lameness was accompanied by decreased appetite and activity.
Cats
Marbofloxacin was administered for 42 consecutive days to 24 cats approximately 8 months old (8 cats per treatment group) at the dosages of 2.5, 7.5 and 12.5 mg/lb/day (5.5, 16.5 and 27.5 mg/kg/day). Treatment with marbofloxacin did not produce adverse effects on body weights, food consumption, serum chemistry, urinalysis or organ weight parameters. Decreased segmented neutrophil counts were observed in some cats in all treatment groups, including the placebo group, but mean counts were significantly lower in the marbofloxacin-treated groups. In some cats, absolute neutrophil counts were below normal reference values (as low as 615 neutrophils/μL in a marbofloxacin-treated cat and as low as 882 neutrophils/μL in a placebo-treated cat). Other hematological observations were not adversely affected. Clinical signs were occasionally noted in cats in the highest dosage group: excessive salivation in 4/8 cats and redness of ear pinnae in 2/8 cats. Macroscopic changes in the articular cartilage of femurs were seen in one cat receiving 7.5 mg/lb and in 3 cats receiving 12.5 mg/lb. Microscopically, these gross lesions were related to a focal or multifocal chondropathy. Microscopic chondropathy not associated with macroscopic observations was also present in one cat treated with 2.5 mg/lb daily (1X the upper end of the dose range) and one additional cat treated with 7.5 mg/lb daily. There was no evidence of lameness during the course of the study. A perivascular to diffuse dermatitis was seen microscopically in one mid-dose cat and 4 high-dose cats. Funduscopic exam by a board-certified ophthalmologist and histologic examination of retina and optic nerve by ocular pathologists revealed no lesions in any of the treatment groups.
Marbofloxacin was also administered orally to 6 cats approximately 8 months of age for 14 consecutive days at a dosage of 25 mg/lb/day (55 mg/kg/day). Clinical signs associated with drug intolerance were excessive salivation in 5/6 cats and redness of ear pinnae in all cats after 8 days of treatment. Emesis was noted occasionally in several cats and diminished activity was noted in one cat. Decreased food intake was noted in some animals, primarily males, when compared to controls. Perivascular to diffuse dermatitis was seen microscopically in the pinnae of all treated animals and in the standard skin samples of several animals. There was focal or multifocal articular chondropathy in 2/6 treated animals. One treated cat had a duodenal mucosal erosion and one treated cat had a pyloric ulcer. There were no observations of lameness and no adverse effects on hematology, clinical chemistry, urinalysis, or organ weight parameters. Funduscopic examination by a board-certified ophthalmologist and histologic examination of retina and optic nerve by ocular pathologists revealed no lesions.
A study was conducted to investigate the effect of marbofloxacin on articular cartilage of skeletally mature cats 12–14 months of age. Forty cats were randomly assigned to 4 groups of 10 cats each. Groups received placebo or marbofloxacin at dosages of 1.25, 3.75 or 7.5 mg/lb/day (2.75, 8.25 or 16.5 mg/kg/day) for 42 consecutive days. There were no treatment-related pathological changes in the joints or other tissues. Emesis and soft stools was noted in all treatment groups, including placebo, and increased in frequency with increasing dose and duration of treatment. Emesis was more apparent in the high-dose males.
Storage Conditions
Store below 30°C (86°F).
How Supplied
Zeniquin tablets are available in the following strengths of marbofloxacin and bottle sizes:
25 mg scored tablets supplied in bottles that contain 100 or 250 tablets50 mg scored tablets supplied in bottles that contain 100 or 250 tablets100 mg scored tablets supplied in a 50 tablet bottle200 mg scored tablets supplied in a 50 tablet bottle
References
1. Schneider M, et al: Pharmacokinetics of marbofloxacin in dogs after oral and parenteral administration. J Vet Pharmacol Therap 19:56–61, 1996.
To report suspected adverse effects, and/or obtain a copy of the MSDS, call 1-888-963-8471.
Approved by FDA under NADA # 141-151
zoetis
Distributed by:Zoetis Inc.Kalamazoo, MI 49007
40027358 Revised: May 2019
Principal Display Panel - 25 Mg Bottle Label
Zeniquin® (marbofloxacin)
Tablets
For oral use in dogs and cats only
Antibacterial
Caution: Federal law restrictsthis drug to use by or on theorder of a licensed veterinarian.
Federal law prohibits theextralabel use of this drugin food-producing animals.
25 mg
100 Tablets
Approved by FDA under NADA # 141-151
zoetis

Principal Display Panel - 50 Mg Bottle Label
Principal Display Panel - 100 Mg Bottle Label
Principal Display Panel - 200 Mg Bottle Label
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site