APAP 325 MG hydrocodone bitartrate 5 MG Oral Tablet
INDICATIONS AND USAGE Hydrocodone bitartrate and acetaminophen tablets, USP are indicated for the relief of moderate to moderately severe pain.
American Health Packaging
Related Pills
APAP 325 MG hydrocodone bitartrate 5 MG Oral Tablet
American Health Packaging
hydrocodone bitartrate and acetaminophen tablet
amneal pharmaceuticals of new york llc
hydrocodone bitartrate and acetaminophen tablet
amneal pharmaceuticals of new york llc
APAP 325 MG hydrocodone bitartrate 5 MG Oral Tablet
Rebel Distributors Corp
APAP 325 MG hydrocodone bitartrate 5 MG Oral Tablet
Rebel Distributors Corp
APAP 325 MG hydrocodone bitartrate 5 MG Oral Tablet
Rebel Distributors Corp
APAP 325 MG hydrocodone bitartrate 5 MG Oral Tablet
Rebel Distributors Corp
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site








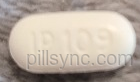




HOW SUPPLIED Hydrocodone Bitartrate and Acetaminophen Tablets, USP, contain hydrocodone bitartrate 5 mg and acetaminophen 325 mg. They are supplied as white to off-white, scored, oblong biconvex tables, debossed "IP 109" on obverse and bisected on the reverse. They are available as follows: Unit dose packages of 100 (10x10) NDC 68084-368-01 Storage: Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°) [see USP Controlled Room Temperature]. A Schedule CIII Narcotic. PACKAGING INFORMATION American Health Packaging unit dose blisters (see How Supplied section) contain drug product from Amneal Pharmaceuticals, LLC as follows: (5 mg / 325 mg / 100 UD) NDC 68084-368-01 packaged from NDC 53746-109. Packaged and Distributed by: American Health Packaging Columbus, OH 43217 8236801/1011
More pills like oval IP 109