LYSODREN (mitotane 500 mg) Dailymed
Generic: mitotane
Boxed Warning
Warning: Adrenal Crisis In The Setting Of Shock Or Severe Trauma
Go PRO for all pill images
Warning: Adrenal Crisis In The Setting Of Shock Or Severe Trauma
In patients taking LYSODREN, adrenal crisis occurs in the setting of shock or severe trauma and response to shock is impaired. Administer hydrocortisone, monitor for escalating signs of shock and discontinue LYSODREN until recovery [see Dosage and Administration (2.2) and Warnings and Precautions (5.1)].
WARNING: ADRENAL CRISIS IN THE SETTING OF SHOCK OR SEVERE TRAUMA
See full prescribing information for complete boxed warning.
In patients taking LYSODREN, adrenal crisis occurs in the setting of shock or severe trauma and response to shock is impaired. Administer hydrocortisone, monitor for escalating signs of shock and discontinue LYSODREN until recovery.(2.2 ,Â5.1)
1 Indications And Usage
LYSODREN is indicated for the treatment of patients with inoperable, functional or nonfunctional, adrenal cortical carcinoma.
LYSODREN is an adrenal cytotoxic agent indicated for the treatment of inoperable, functional or nonfunctional, adrenal cortical carcinoma.(1)
2 Dosage And Administration
•Initial dose: 2 g to 6 g orally daily, in three or four divided doses.(2.1)
•Increase dose incrementally to achieve a blood concentration of 14 to 20 mg/L, or as tolerated.(2.1)
2.1 Recommended Dose
The recommended initial dose of LYSODREN is 2 g to 6 g orally, in three or four divided doses per day. Increase doses incrementally to achieve a blood concentration of 14 to 20 mg/L, or as tolerated.
LYSODREN is a cytotoxic drug. Follow applicable special handling and disposal procedures.
2.2 Dose Modifications
Adrenal Crisis in the Setting of Shock or Severe Trauma
Discontinue LYSODREN until recovery [see Warnings and Precautions (5.1)].
Central Nervous System (CNS) Toxicity
Discontinue LYSODREN until symptoms resolve. Seven to 10 days after symptoms resolve, restart at a lower dose (for example, decrease by 500-1000 mg) [see Warnings and Precautions (5.2)].
3 Dosage Forms And Strengths
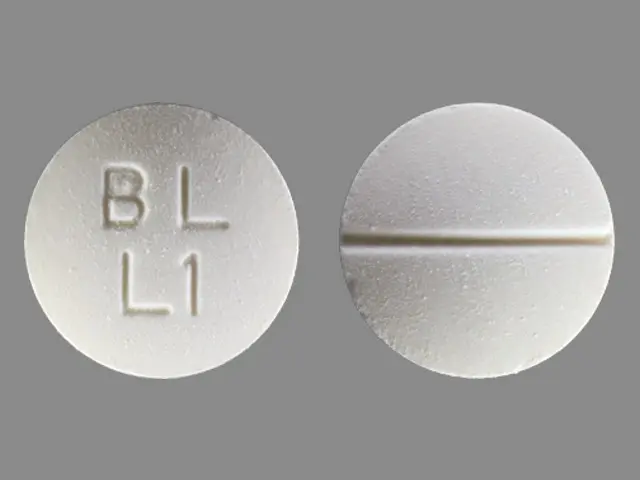
500 mg white, round, biconvex, scored tablets, bisected on one side and impressed with “BL” over “L1” on the other side.
Tablets: 500 mg, scored(3)
4 Contraindications
None.
None(4)
5 Warnings And Precautions
- Central Nervous System (CNS) Toxicity: Plasma concentrations exceeding 20 mcg/mL are associated with a greater incidence of toxicity.
(5.2) - Adrenal Insufficiency: Institute steroid replacement as clinically indicated. Measure free cortisol and corticotropin (ACTH) levels to achieve optimal steroid replacement.
(5.3) - Embryo-Fetal Toxicity: Can cause fetal harm. Advise women of reproductive potential of the potential risk to a fetus and use of effective contraception. (
5.4 ,8.1 ,8.3 )- Ovarian Macrocysts in Premenopausal Women: Advise women to seek medical advice if they experience gynecological symptoms such as vaginal bleeding and/or pelvic pain. (
5.5 )5.1 Adrenal Crisis in the Setting of Shock or Severe Trauma
In patients taking LYSODREN, adrenal crisis occurs in the setting of shock or severe trauma and response to shock is impaired. Administer hydrocortisone, monitor for escalating signs of shock, and discontinue LYSODREN until recovery [see Dosage and Administration (2.2)].
5.2 CNS Toxicity
CNS toxicity, including sedation, lethargy, and vertigo, occurs with LYSODREN treatment. Mitotane plasma concentrations exceeding 20 mcg/mL are associated with a greater incidence of toxicity.
5.3 Adrenal Insufficiency
Treatment with LYSODREN can cause adrenal insufficiency. Institute steroid replacement as clinically indicated. Measure free cortisol and corticotropin (ACTH) levels to achieve optimal steroid replacement.
5.4 Embryo-Fetal Toxicity
LYSODREN can cause fetal harm when administered to a pregnant woman. Abnormal pregnancy outcomes, such as preterm births and early pregnancy loss, can occur in patients exposed to mitotane during pregnancy. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with LYSODREN and after discontinuation of treatment for as long as mitotane plasma levels are detectable [see Use in Specific Populations (8.1, 8.3)].
5.5 Ovarian Macrocysts in Premenopausal Women
Ovarian macrocysts, often bilateral and multiple, have been reported in premenopausal patients receiving LYSODREN. Complications from these cysts, including adnexal torsion and hemorrhagic cyst rupture, have been reported. In some cases, improvement after mitotane discontinuation has been described. Advise female patients to seek medical care if they experience gynecological symptoms such as vaginal bleeding and/or pelvic pain [see Adverse Reactions (6)].
6 Adverse Reactions
The following adverse reactions are discussed in greater detail in other sections of the label:
- Adrenal Crisis in the Setting of Shock or Severe Trauma [see Warnings and Precautions (5.1)]
- CNS Toxicity [ see Warnings and Precautions (5.2)]
- Adrenal Insufficiency [see Warnings and Precautions (5.3)]
- Ovarian macrocysts [seeWarnings and Precautions (5.5)]
The following adverse reactions associated with the use of LYSODREN were identified in clinical trials or postmarketing reports. Because these reactions were reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency reliably or to establish a causal relationship to drug exposure.
Common adverse reactions occurring with LYSODREN treatment include:
- Anorexia, nausea, vomiting, and diarrhea (80%)
- Depression, dizziness, or vertigo (15%-40%)
- Rash (15%)
- Neutropenia
- Growth retardation, hypothyroidism
- Confusion, headache, ataxia, mental impairment, weakness, dysarthria
- Maculopathy
- Hepatitis, elevation of liver enzymes
- Gynecomastia
- Hypercholesterolemia, hypertriglyceridemia
- Decreased blood androstenedione and decreased blood testosterone in females, increased sex hormone binding globulin in females     and males, decreased blood free testosterone in males.
Less common adverse reactions include: visual blurring, diplopia, lens opacity, retinopathy, prolonged bleeding time, hematuria, hemorrhagic cystitis, albuminuria, hypertension, orthostatic hypotension, flushing, generalized aching, and fever.
Common adverse reactions (≥15%) include: anorexia, nausea, vomiting and diarrhea; depression, dizziness or vertigo; and rash.(6)
To report SUSPECTED ADVERSE REACTIONS, contact Bristol-Myers Squibb at 1-800-721-5072 or FDA at 1-800-FDA-1088 orwww.fda.gov/medwatch.
7 Drug Interactions
Adjust dosage of concomitant coumarin-type anticoagulants as needed.(7.2)
7.1 CYP3A4 Substrates
Mitotane is a strong inducer of cytochrome P450 3A4 (CYP3A4). Monitor patients for a change in dosage requirements for the concomitant drug when administering LYSODREN to patients receiving drugs that are substrates of CYP3A4.
7.2 Warfarin
When administering coumarin-type anticoagulants to patients receiving LYSODREN, monitor coagulation tests and adjust the anticoagulant dose as needed.
8 Use In Specific Populations
• Lactation: Do not breastfeed.(8.2) • Hepatic Impairment: Administer LYSODREN with caution to patients with hepatic impairment.(8.6) 8.1 Pregnancy
Risk Summary
LYSODREN can cause fetal harm. Limited postmarketing cases report preterm births and early pregnancy loss in women treated with LYSODREN during pregnancy. Animal reproduction studies have not been conducted with mitotane. Advise pregnant women of the potential risk to a fetus. The background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
8.2 Lactation
Risk Summary
Mitotane is excreted in human milk; however, the effect of LYSODREN on the breastfed infant, or effect on milk production is unknown. Because of the potential for serious adverse reactions in the breastfed infant, advise nursing women that breastfeeding is not recommended during treatment with LYSODREN and after discontinuation of treatment for as long as mitotane plasma levels are detectable.
8.3 Females and Males of Reproductive Potential
Contraception
Females
LYSODREN can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].ÂAdvise female patients of reproductive potential to use effective contraception during treatment with LYSODREN and after discontinuation of therapy for as long as mitotane plasma levels are detectable [see Clinical Pharmacology (12.3)].
8.4 Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
8.5 Geriatric Use
Clinical studies of LYSODREN did not include sufficient numbers of patients aged 65 years and older to determine whether they respond differently than younger patients. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
8.6 Hepatic Impairment
Hepatic impairment may interfere with the metabolism of mitotane and the drug may accumulate. Administer LYSODREN with caution to patients with hepatic impairment.
11 Description
LYSODREN (mitotane) is an oral adrenal cytotoxic agent. The chemical name is (±)-1,1-dichloro-2-(o-chlorophenyl)-2-(p-chlorophenyl) ethane (also known as o,p′-DDD). The chemical structure is:
![]()
Mitotane is a white granular solid composed of clear colorless crystals. It is tasteless and has a slight pleasant aromatic odor. It is soluble in ethanol and has a molecular weight of 320.05.
Inactive ingredients in LYSODREN are: microcrystalline cellulose, polyethylene glycol 3350, silicon dioxide, and starch.
12 Clinical Pharmacology
12.1 Mechanism of Action
Mitotane is an adrenal cytotoxic agent with an unknown mechanism of action. Mitotane modifies the peripheral metabolism of steroids and directly suppresses the adrenal cortex. A reduction in 17-hydroxycorticosteroids in the absence of decreased corticosteroid concentrations and increased formation of 6-β-hydroxycortisol have been reported.
12.2 Pharmacodynamics
The pharmacodynamics of mitotane are unknown.
12.3 Pharmacokinetics
Absorption
Following oral administration of LYSODREN, 40% of the dose is absorbed.
Distribution
Mitotane is found in most tissues of the body; however, fat is the primary site of distribution.
Elimination
Following discontinuation of mitotane, the plasma terminal half-life ranges from 18 to 159 days (median 53 days).
Metabolism
Mitotane is converted to a water-soluble metabolite.
Excretion
No unchanged mitotane is found in urine or bile. Approximately 10% of the administered dose is recovered in the urine as a water-soluble metabolite. A variable amount of metabolite (1%-17%) is excreted in the bile.
13 Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
The carcinogenicity and mutagenicity of mitotane are unknown.
15 References
1.  OSHA. http://www.osha.gov/SLTC/hazardousdrugs/index.html
16 How Supplied/storage And Handling
LYSODREN tablets are supplied as 500 mg white, round, biconvex, scored tablets, bisected on one side and impressed with “BL” over “L1” on the other side.
100 tablets per bottle: NDC 0015-3080-60
Store bottles at 25°C (77°F); excursions permitted between 15°C and 30°C (59°F-86°F).
Mitotane is a cytotoxic drug. Follow applicable special handling and disposal procedures [see References (15)].
17 Patient Counseling Information
Adrenal Crisis
- Advise patients to discontinue LYSODREN in the case of shock or severe trauma and contact their healthcare provider immediately.
- Advise patients to tell their healthcare provider of any planned surgeries.
Ovarian Macrocysts
- Advise premenopausal women to seek medical care if they experience gynecological symptoms such as vaginal bleeding and/or pelvic pain [see Warnings and Precautions (5.5) ].
Embryo-Fetal Toxicity
- Advise females of reproductive potential of the potential risk to a fetus and to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.4) and Use in Specific Populations (8.1)].
- Advise females of reproductive potential to use effective contraception during treatment and after discontinuation of treatment for as long as instructed by their healthcare provider [see Use in Specific Populations (8.3)].
Lactation
- Advise females who are nursing not to breastfeed during treatment with LYSODREN [see Use in Specific Populations (8.2)].
Manufactured for:Bristol-Myers Squibb CompanyPrinceton, New Jersey 08543 USA Revised: November 2019
Lysodren 500 Mg Tablets Representative Packaging
See How Supplied section for a complete ul of available packages of LYSODREN.
NDC 0015-3080-60 100 TABLETS LYSODREN® (mitotane tablets, USP) EACH TABLET CONTAINS 500 mg Rx only Bristol-Myers Squibb
![]()
DISCLAIMER:
"This tool does not provide medical advice, and is for informational and educational purposes only, and is not a substitute for professional medical advice, treatment or diagnosis. Call your doctor to receive medical advice. If you think you may have a medical emergency, please dial 911."
"Do not rely on openFDA to make decisions regarding medical care. While we make every effort to ensure that data is accurate, you should assume all results are unvalidated. We may limit or otherwise restrict your access to the API in line with our Terms of Service."
"This product uses publicly available data from the U.S. National Library of Medicine (NLM), National Institutes of Health, Department of Health and Human Services; NLM is not responsible for the product and does not endorse or recommend this or any other product."
PillSync may earn a commission via links on our site